Why is a glass vessel with a narrow neck (narrow-neck flask) or a wider neck (wide-neck flask) called an Erlenmeyer flask?
For a long time, I was not even aware that the name had anything to do with a person—namely, Richard August Carl Emil Erlenmeyer, who was born 200 years ago. Did he often get annoyed when swirling a beaker because some of the liquid splashed out, leading him to pursue this design?
Biographical Information
Erlenmeyer was born on June 28, 1825, in Wehen (today Taunusstein), Germany [1–6]. From 1835 to 1839, he attended the Pädagogium (a preparatory school) in Wiesbaden, Germany. Then, private tutors prepared him for the final examination (Maturitätsexamen), which he successfully passed in 1845. He decided to study medicine and therefore went to Gießen, Germany. The lectures of Justus (von) Liebig (1803–1873) impressed him so much that he turned to chemistry. However, Liebig’s laboratory was in such high demand that Erlenmeyer could not get a place.
As a result, he went to Heidelberg, Germany, where he primarily attended lectures on physics, botany, and mineralogy. When he returned to Gießen in 1847, he still could not secure a spot in Liebig’s laboratory, but he was given a workplace and, one semester later, an assistant position under Liebig’s student Heinrich Will (1812–1890), who managed Liebig’s satellite laboratory on Seltersberg near Gießen.
 Will recommended that Erlenmeyer pursue an academic career, but he simply lacked the financial means. Presumably at his father’s explicit request, Erlenmeyer took the pharmaceutical state examination against his own wishes and acquired a pharmacy in Katzenelnbogen,Germany, in 1849, only to sell it again one year later.
Will recommended that Erlenmeyer pursue an academic career, but he simply lacked the financial means. Presumably at his father’s explicit request, Erlenmeyer took the pharmaceutical state examination against his own wishes and acquired a pharmacy in Katzenelnbogen,Germany, in 1849, only to sell it again one year later.
With his dissertation “On Basic Lead Cyanide”, Erlenmeyer earned his doctorate under Liebig in 1850. He then purchased a pharmacy in Wiesbaden,Germany, where he also taught chemistry at the local commercial and industrial school. However, he did not remain dedicated to this pharmacy for long either—he sold it after five years, probably because his attempt to expand it into a chemical enterprise had failed.
He moved back to Heidelberg and set up a small research laboratory at Karpfengasse 6. With a study on artificial fertilizers, he qualified as a lecturer (Habilitation) in 1857 under Robert Bunsen (1811–1899) and was appointed a Privatdozent (private lecturer). In 1856, Friedrich August Kekulé (1829–1896) had also completed his Habilitation in Heidelberg, allowing him to hold lectures that were attended not only by Adolf (von) Baeyer (1835–1917) and Aleksandr M. Butlerov (1828–1886) but also by our celebrant, Erlenmeyer.
In 1859, Kekulé was called to Ghent, Belgium, and Bunsen lost interest in modern aspects of organic chemistry. Heidelberg University had a very good reputation in Russia, so many Russians came there for a few semesters of study. Erlenmeyer’s laboratory was the workplace of many budding Russian chemists. Among those were Aleksandr P. Borodin (1833–1887), who is also known as a composer, and Vladimir V. Markovnikov (1838–1904).
Erlenmeyer also became acquainted with Dmitri I. Mendeleev (1834–1907), who conducted physicochemical investigations in his own small laboratory in Heidelberg but preferred to use Erlenmeyer’s lab for larger and more hazardous experiments. It is said that Erlenmeyer charged a fee for using his lab that was twice as high as that of the university’s laboratory. Nonetheless, researchers continued to come [7].
Erlenmeyer’s lab was not only used for training but also for industrial consulting, which provided the necessary funding to maintain the private facility. Erlenmeyer was a co-owner of a small chemical factory that produced an unusual range of products, including potash, axle grease, leather, shellac, black varnishes, must preservatives, dyes for coloring wine, as well as rum, punch, and fruit essences [8].
In 1863, Emil Erlenmeyer was appointed an associate professor. In addition to his lectures on experimental organic chemistry, with a special focus on medicine and pharmacy, he also held pharmaceutical-chemical practical courses. In 1868, he accepted a position at the newly established Polytechnic School (Technische Hochschule, TH) in Munich, Germany, where he served as director from 1877 to 1880. (From 1877, it was called the Royal Bavarian Technical University of Munich, and it was renamed the Technical University in 1970.)
However, the lack of the right to confer doctoral degrees at technical universities was a disadvantage. As a result, students would train under Erlenmeyer but then transfer to a university for their degrees. Despite this, students eagerly attended his lectures because Liebig and Jacob Volhard (1834–1910) still taught outdated theories of dualism and Charles Frédéric Gerhardt‘s theory of types. The theory of types, claims that classes of similar organic compounds could be derived from, e.g., water or ammonia by adding different residues. In this way, alcohols and ethers belonged to a “water type”, amines to the “ammonia type”, etc. This classification system was a foundation for organic structure theory, later developed by August Kekulé.
In 1883, Erlenmeyer left Munich and moved to Frankfurt (Main), Germany, relocating to Wiesbaden two years later, and then back to Frankfurt in 1886. He joined the consulting firm of his former student Ludwig Belli (1852–1904) and conducted research for the Casella company. Founded in 1798 by Leopold Cassella (1766–1847) in Frankfurt, the chemical and pharmaceutical company operated independently until 1995, eventually becoming a predecessor of Sanofi. From 1893, Erlenmeyer lived in Aschaffenburg, Germany, and worked at the forestry academy’s laboratory until 1897.
Erlenmeyer as a Theorist of Organic Chemistry
Erlenmeyer believed that it was necessary to establish a universal theoretical foundation for organic chemistry. As early as the 1860s, he used the term valency to describe the number of bonds an atom can form. This concept was built upon the groundwork laid by Sir Edward Frankland (1825–1899), who had developed the idea of saturation capacity.
It is somewhat surprising that Lothar Meyer (1830–1895), who was deeply engaged with modern chemical theories, did not mention in his foundational work that the terms valency and valence, still in use today, originated with Erlenmeyer [9]. Both Erlenmeyer and Butlerov introduced the concept of structural chemistry [10].
However, Erlenmeyer did not adopt the tetrahedral model of the carbon atom, which was based on William H. Wollaston’s (1766–1828) 1808 ideas on five-atom molecules and was later championed by Archibald Scott Couper (1831–1892) and Kekulé. Erlenmeyer could not reconcile this model with the different reaction conditions observed in methane substitution reactions.
Nevertheless, he also recognized the tetravalency of carbon as the guiding principle for constructing chemical formulas. In 1858, Kekulé and Couper used chain models to represent molecules, but these did not gain widespread acceptance. Instead, Alexander Crum Brown (1838–1922) proposed line symbols in 1861, which Frankland popularized in lectures. Couper had also developed such line formulas. Erlenmeyer adopted this notation and systematically used it to depict the double bond in ethene and the triple bond in ethyne. The classification of multiple bonds as a general principle is attributed to Erlenmeyer [11,12].
Erlenmeyer as a Practical Organic Chemist
Erlenmeyer discovered and synthesized isobutyric acid, determined the correct formulas for guanidine, creatine, and creatinine, synthesized tyrosine and various hydroxycarboxylic acids, and established that lactones are internal esters of γ-hydroxycarboxylic acids. He elucidated the structure of eugenol and demonstrated that its oxidation produces vanillin.
In 1880, he observed that alcohols with hydroxyl groups attached to double bonds are unstable and undergo transformation into aldehydes and ketones (see Fig. 1).
He also determined that a single carbon atom cannot bear multiple unsubstituted hydroxyl groups. This principle is still known today as Erlenmeyer’s Rule. The rule is still used as a general guideline, but it is known that in certain cases, geminal hydroxyl groups can be stable, especially when electron-withdrawing substituents are adjacent (e.g., in Ninhydrin or Chloral hydrate). In such cases, the unstable interaction between the hydroxyl groups is stabilized by the neighboring substituents.
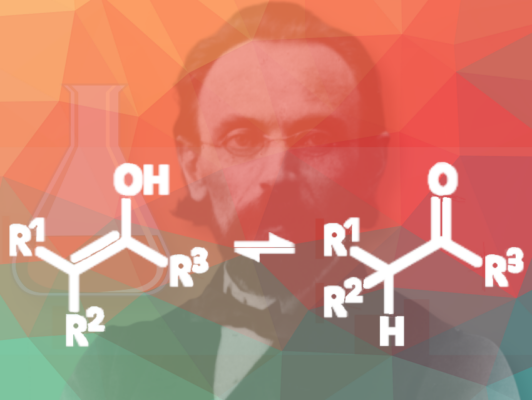
Figure 1. Erlenmeyer observed that alcohols with an OH group attached to a double-bonded carbon become aldehydes or ketones. They do this because these compounds are generally more stable than the alcohol-containing tautomer. This idea is today referred to as keto-enol tautomerism.
The term “hydroxy(l) group” is also said to originate from Erlenmeyer [13].
He paid relatively little attention to dyes, even though they were a major focus in chemistry at the time. He wrote an analysis of the structure of methylene blue and held a patent for the production of dyes in the rosaniline series.
Erlenmeyer as Author and Editor
In 1868, Erlenmeyer undertook the project of writing a textbook on organic chemistry. His structural theoretical views formed the basis for its classification. Each compound was to be thoroughly and meticulously described. However, the project remained unfinished, as the number of known organic compounds was rapidly increasing at the time, and Friedrich K. Beilstein (1838–1906) later provided a more efficient solution for managing this diversity with his “Handbook of Organic Chemistry”.
Erlenmeyer was also involved as an editor in various scientific journals. In 1859, Kekulé founded the Critical Journal for Chemistry, Pharmacy, and Mathematics, which, after Kekulé moved to Ghent, was continued by Erlenmeyer and Gustav Lewinstein (1830–1902). However, Lewinstein soon withdrew—possibly due to a dispute between the two? Erlenmeyer continued the journal alone.
Erlenmeyer’s often harsh and scathing comments offended many authors and likely some readers as well. His jokes were reportedly so coarse that they were deemed unprintable. Eventually, in 1865, he had to relinquish the journal, as the number of subscribers had dwindled to just 150—half of whom were Russian. Erlenmeyer proposed that Butlerov transform the journal into a Russian publication, but this suggestion found no support. Eventually, Friedrich K. Beilstein, Rudolph Fittig (1835–1910), and Hans Hübner (1837–1884) continued publishing the journal until 1871.
Despite this, in 1871, on the recommendation of Jacob Volhard, Liebig appointed Erlenmeyer as one of the editors of the Annalen der Chemie. This decision had a significant impact, particularly regarding crucial contributions to the periodic system by Lothar Meyer and Dmitri Mendeleev. At the time, Hermann Kopp, who was skeptical of speculative works, was still responsible for the Annalen, which led Lothar Meyer to submit his important paper, “The Nature of Chemical Elements as a Function of Their Atomic Weights”, in a highly condensed form [14]. As Meyer himself later noted, he was forced to keep his contribution brief [15], whereas Mendeleev was given much more space shortly afterward [16].
There were numerous concerns about Erlenmeyer’s appointment as editor because he had made himself unpopular. In response, Kekulé proposed to the German Chemical Society that the society’s newsletter, Reports of the German Chemical Society in Berlin, be transformed into a full-fledged chemistry journal, aiming to establish independence from the critical Erlenmeyer. To protect the Annalen from potential harm, Erlenmeyer brought Kekulé and August Wilhelm von Hofmann (1818–1892) onto the editorial board. Although conflicts eventually arose between Hofmann and Erlenmeyer, the journal itself did not suffer under Erlenmeyer’s leadership.
Annalen der Chemie is still in circulation today under the name European Journal of Organic Chemistry.
Erlenmeyer as a University Lecturer
In the laboratory, Erlenmeyer placed great emphasis on strict order and cleanliness in the workrooms. Violations were subject to fines.
He was also interested in teaching methods—what we would today call university didactics. In a speech delivered at the public session of the Royal Academy of Sciences in Munich on July 25, 1871, he presented his ideas on chemical education. Regarding laboratory instruction, he argued that a considerable number of instructors were necessary for successful guidance. He further stated [17]:
“Practical exercises in the laboratory must be accompanied by the most intensive mental exercises, for nothing is as difficult yet as necessary as training aspiring chemists to think independently. Beginners too easily fall into mindless, rote procedures; they read, but they do not study; they handle materials, but they do not experiment; they look, but they do not observe. If the instructor does not continuously engage with them and maintain their aware-ness through constant questioning, they will not be encouraged—or even forced—to reflect on their work.”
Erlenmeyer also contributed to improving laboratory techniques. In addition to the Erlenmeyer flask (see Fig. 2), his achievements included introducing asbestos nets—formerly used in chemistry labs as heat-resistant supports for glassware over Bunsen burners but now banned due to health risks—designing more efficient combustion furnaces for CH analysis, and constructing ovens for heating closed tubes.

Figure 2. The Erlenmeyer flask (also known as a conical flask) has a flat bottom, a conical body that narrows towards the top, and a cylindrical neck. It was developed by Emil Erlenmeyer in 1860.
The illustration is attributed to Erlenmeyer, though this is not certain, and is believed to date to 1861. (public domain)
To improve chemistry education in schools, Erlenmeyer considered it essential to enhance teacher training. As a result, he introduced weekly two-hour practical exercises for teacher candidates, during which they had to conduct experimental lectures. Notably, Erlenmeyer would immediately interrupt candidates if he noticed a linguistic or experimental mistake.
Reports indicate that Erlenmeyer’s lectures were not particularly smooth, but his enthusiasm and liveliness made them captivating [18]:
“He did not seek to impress with elaborate rhetorical flourishes or an excess of dazzling experiments. Instead, through simple, well-prepared demonstrations and clear definitions, complemented by historical and critical explanations, he was able to convince his audience.”
So the next time you hold an Erlenmeyer flask or explain the Erlenmeyer rule, remember the 200th birthday of its inventor!

Figure 3. Erlie, inspired by the Erlenmeyer flask, is the mascot of the German Chemical Society (GDCh), especially loved by the Young Chemists Network (JCF). (Photo: V. Koester)
References
[1] Rita Meyer: Emil Erlenmeyer (1825–1909) als Chemietheoretiker und sein Beitrag zur Entwicklung der Strukturchemie, Diss., Institut für Geschichte der Medizin der Universität München, 1984.
[2] Heinrich Kiliani: Dem Andenken von Emil Erlenmeyer, Zeitschrift für angewandte Chemie und Zentralblatt für technische Chemie 1909, 22, 481–483.
[3] Max Conrad: Emil Erlenmeyer, Berichte der Deutschen Chemischen Gesellschaft 1910, 43, 3645–3664. https://doi.org/10.1002/cber.191004303163
[4] Otto Krätz: Das Portrait: Emil Erlenmeyer 1825–1909, Chemie in unserer Zeit 1972, 6, 43–58. https://doi.org/10.1002/ciuz.19720060204
[5] Claus Priesner: Emil Erlenmeyer, Lexikon der bedeutenden Naturwissenschaftler (Hrsg. von Dieter Hoffmann; Hubert Laitko et al.), Heidelberg, Berlin: Spektrum Akademischer Verlag, 2003, 1. Band, 481–482.
[6] Gisela Boeck: Zum Gedenken: Richard August Carl Emil Erlenmeyer, ChemKon 2009, 16, 105–106. https://doi.org/10.1002/ckon.200990011
[7] Otto Krätz: Iwan Turgenjew und die russischen Chemiker in Heidelberg, Chemie in unserer Zeit 1987, 21, 89–99. https://doi.org/10.1002/ciuz.19870210304
[8] Krätz (1972), 54 bzw. 58.
[9] Lothar Meyer: Die modernen Theorien der Chemie und ihre Bedeutung für die chemische Statik, Breslau: Maruschke & Berendt 1864. Siehe auch: Gisela Boeck, Alan J. Rocke: Lothar Meyer: Modern Theories and Pathways to Periodicity, Cham: Springer International Publishing AG, 2022.
[10] Otto Krätz: Beilstein Erlenmeyer Briefe zur Geschichte der chemischen Dokumentation und des chemischen Zeitschriftenwesens, München: Werner Fritsch, 1972, hierzu vgl. beson-ders S. 12–13.
[11] Vergleiche dazu z. B. Ursula Klein: Experiments, models, paper tools: Cultures of organ-ic chemistry in the nineteenth Century, Stanford: Stanford University Press 2003.
[12] Zu Fragen der Strukturtheorie siehe: Alan J. Rocke: The quiet revolution: Hermann Kolbe and the science of organic chemistry, Berkeley [u.a.] : Univ. of California Press, 1993.
[13] Conrad (1910), 3655.
[14] Lothar Meyer: Die Natur der chemischen Elemente als Function ihrer Atomgewichte,
Annalen der Chemie und Pharmacie, Suppl.-Bd. 7, 354–64.
[15] Meyer am 13. November 1886 an Clemens Winkler (Winkler Nachlass, Universitätsbib-liothek der Technischen Universität Bergakademie Freiberg)
[16] Dmitri I. Mendeleev: Die periodische Gesetzmässigkeit der chemischen Elemente, An-nalen der Chemie und Pharmacie, Suppl.-Bd. 8, Nr. 2, 1871, 133–229.
[17] Emil Erlenmeyer, Die Aufgaben des chemischen Unterrichts gegenüber den Anforde-rungen der Wissenschaft und Technik, München: Verlag der Königlichen Akademie 1871, S. 24.
[18] Conrad (1910) 3648/49.
Emil Erlenmeyer is the answer to Guess the Chemist (161).
Also of Interest

Which of the following well-known researchers were students of Liebig, and which ones were not?

German scientist (1803 – 1873) described as a founder of organic chemistry and “father of the fertilizer industry”

EuChemS acknowledges significance of Liebig and his laboratory for the development of chemistry




