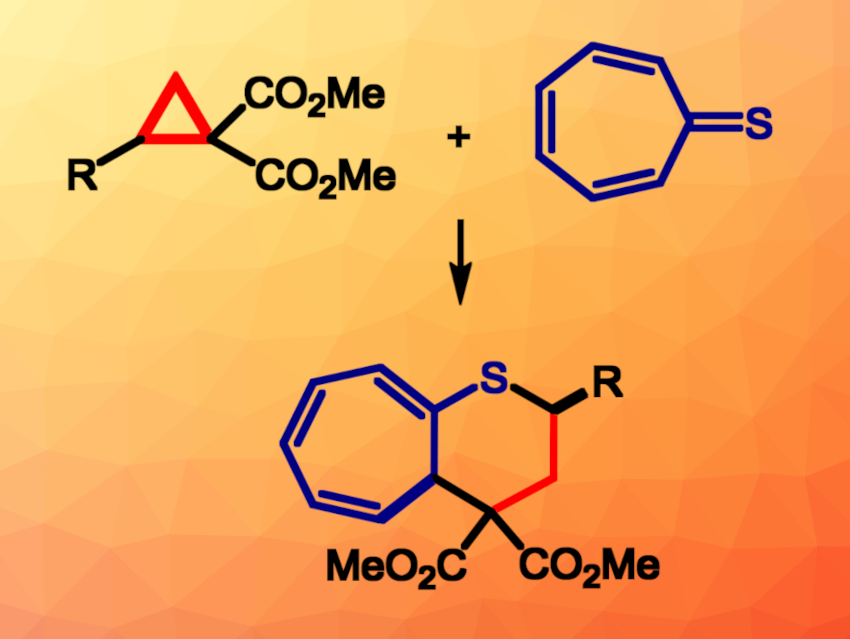
Thioketones can be used for the preparation of five- and six-membered sulfur heterocycles via (3+2)- and (4+2)-cycloadditions, respectively. Thioketones can show high reactivities towards 1,3-dipoles and dienes. Polarized donor-acceptor cyclopropanes (D-A cyclopropanes) can undergo (3+2)-cycloaddition reactions with a variety of dipolarophiles.
Grzegorz Mlostoń, University of Łodź, Poland, and colleagues have developed an approach to the diastereoselective (8+3)-cycloaddition of D-A cyclopropanes with tropothione, the thio analogue of 2,4,6-cycloheptatrien-1-one (general reaction pictured). The team reacted different dimethyl 2-aryl cyclopropane-1,1-dicarboxylates with tropothione in the presence of the Lewis acid Sc(OTf)3, using CH2Cl2 as the solvent. The reactions were performed at room temperature.
The researchers found that tropothione smoothly undergoes (8+3)-cycloadditions with D-A cyclopropanes. The corresponding fused thiopyrans were obtained in moderate to good yields and in a diastereoselective manner. The products were formed as single diastereomers with cis-orientation of the H atoms at the C2 and C5 positions. Overall, the work provides a simple and selective procedure for the synthesis of fused thiopyrans with a troponoid unit, as well as insights into cycloaddition reactions involving lesser explored thiocarbonyl compounds.
- Diastereoselective (8+3)‐Cycloadditions of Donor‐Acceptor Cyclopropanes with Tropothione,
Grzegorz Mloston, Mateusz Kowalczyk, Marcin Palusiak, Gwyndaf A. Oliver, Heinrich F. Koeller, Daniel B. Werz,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202301182
![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
