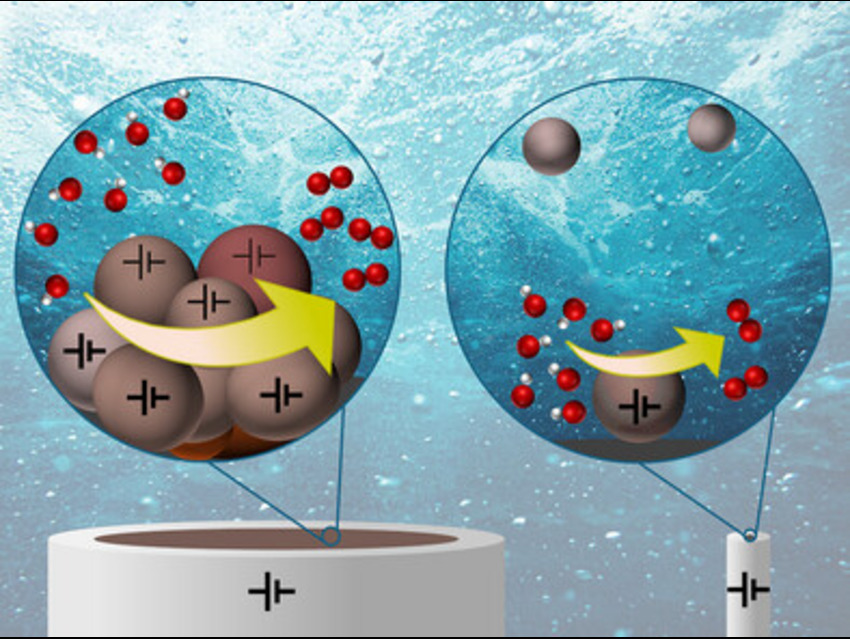
A major challenge in clean energy research is accurately measuring how efficiently tiny catalyst particles promote oxygen evolution (OER) during water splitting. Traditional methods often hide the true performance of these particles because they involve binders and clumped particles, making it hard to see how each one behaves on its own.
Kristina Tschulik, Ruhr-University Bochum, Germany, and colleagues have used a unique technique called nano-impact electrochemistry to study individual catalyst particles in action, without interference from neighboring particles or added materials. This approach gives a clearer picture of how each nanoparticle performs, helping design better catalysts for renewable energy systems like water electrolyzers.
The researchers used cobalt ferrite (CoFe₂O₄) nanoparticles, about 3.5 nm in size, as catalysts for the OER in an alkaline 0.1 M KOH solution. Two types of electrodes, a glassy carbon rotating disk electrode and a carbon ultramicroelectrode, were used across three experimental setups:
- In the drop-casting method, nanoparticles were layered onto the electrode, showing increased activity at low loadings but reduced performance at higher loadings due to poor conductivity and limited access to active sites.
- In the particle sticking method, nanoparticles were gradually adsorbed onto the microelectrode, allowing better spacing and resulting in a steady rise in OER current.
- The most precise approach, nano-impact electrochemistry, involved freely diffusing nanoparticles randomly colliding with the microelectrode, generating distinct current spikes that directly reflected the catalytic activity of individual particles.
The key chemical step was identified as the conversion of surface-bound hydroxide (MOH + OH⁻ → MO⁻ + H₂O), consistent with known OER mechanisms. At high applied potentials, the current from single-particle impacts closely matched theoretical predictions. Crucially, both single nanoparticles and small groups showed similar reaction behavior, with a Tafel slope of ~60 mV/decade, indicating a shared rate-limiting step.
This confirms that nano-impact methods can reliably measure intrinsic catalyst activity and offer a powerful way to study electrocatalysts without interference from binders or particle clustering. This work shows that nano-impact electrochemistry is a powerful tool for studying catalysts at the single-particle level. It avoids the problems of traditional methods and helps researchers understand how each particle contributes to the overall reaction. These insights will guide the development of more efficient, stable, and scalable catalysts for clean energy technologies.
- Probing the Intrinsic Oxygen Evolution Kinetics at Single CoFe2O4 Nano-Catalysts
Hatem M. A. Amin, Mahnaz Azimzadeh Sani, Abdelilah El Arrassi, Sascha Saddeler, Stephan Schulz, Kristina Tschulik
ChemCatChem 2025
https://doi.org/10.1002/cctc.202501234