Water scarcity in Morocco is intensifying due to population growth, economic development, and climate change. To address this challenge, F. Z. Addar and colleagues at Ibn Tofail University, Kenitra, Morocco, investigated how salinity impacts the performance of membrane-based desalination, focusing on nanofiltration (NF) and reverse osmosis (RO) as sustainable solutions for treating brackish water.
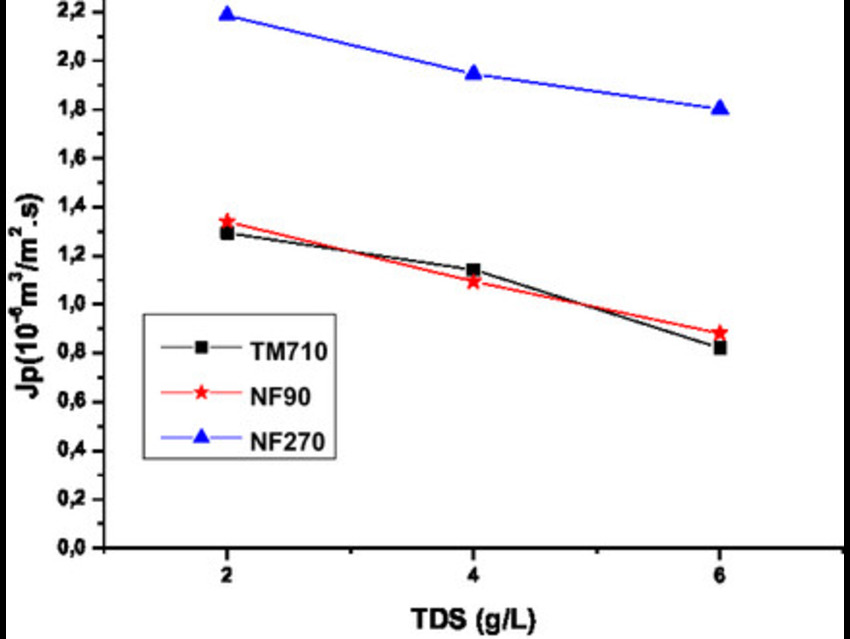
The team evaluated three membranes—NF270, NF90, and TM710—using synthetic brackish water with total dissolved solids of 2, 4, and 6 g/L. They measured permeate flux under varying transmembrane pressures and modeled ion transport using the Spiegler-Kedem and Kedem-Katchalsky equations.
NF270 delivered the highest water flux but showed reduced salt rejection as salinity increased. Its sharp increases in diffusion flux and convective concentration pointed to strong polarization effects and dominant convective transport. TM710, in contrast, maintained consistently high salt rejection and low solute permeability, with reflection coefficients near 1, indicating diffusion-driven ion transfer. NF90 offered a balance of moderate flux and high rejection, performing similarly to TM710 and making it particularly suitable for energy-efficient desalination.
The results suggest that NF90 could replace RO membranes in low-pressure systems, reducing energy consumption while maintaining high salt rejection, whereas NF270 is better suited for applications where water flux is prioritized over salt removal.
- Salinity Effect in Permeability of Salt in Nanofiltration and Reverse Osmosis Membranes
A. Lachheb, A. El Attar, F. Z. Addar, I. Kouda, N. Zouhri, J. Touir, M. Taky, M. Tahaikt
ChemistryOpen 2025
https://doi.org/10.1002/open.202500198