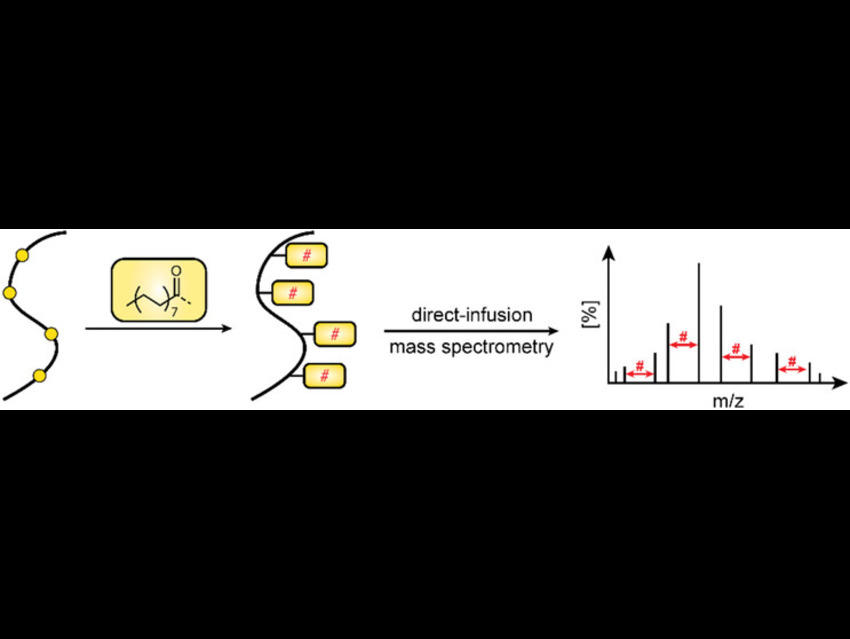
Lipidation refers to the covalent attachment of lipid groups—such as palmitoyl, myristoyl, or prenyl groups—to proteins. Lipidation, particularly S-palmitoylation, is a reversible protein modification that influences membrane association and protein interactions. Yet it remains underexplored in proteomics because lipidated peptides often show poor solubility and low ionization efficiency during mass-spectrometric analysis.
Carla Schmidt, Johannes Gutenberg University Mainz, Germany, and colleagues investigated how multiple S-palmitoylation events influence peptide analysis by mass spectrometry. The team used synaptosome-associated protein 25 (SNAP25) as a model system. SNAP25 is a SNARE protein (a class of membrane fusion proteins) essential for neurotransmitter release. It is anchored to membranes through four S-palmitoylated cysteine residues.
To mimic this natural modification, the team synthesized six peptide variants (P1–P6) from a tryptic SNAP25 fragment containing four cysteines. They selectively introduced S-palmitoylation by blocking specific cysteines with carbamidomethylation, a common proteomics modification. Lipidation was introduced in vitro using palmitoyl chloride in trifluoroacetic acid.
The peptides were analyzed using direct-infusion nanoelectrospray ionization (ESI) MS. Higher-energy collisional dissociation (HCD) fragmentation provided full sequence coverage and confirmed that S-palmitoylation remained intact during tandem MS, allowing precise localization of modification sites.
To improve ionization of doubly palmitoylated peptides, the researchers tested dimethyl sulfoxide (DMSO) as a solvent additive. At 10% DMSO and 1.0 kV ionization voltage, signal intensity doubled, likely due to the formation of smaller charged droplets during ESI.
The study demonstrates that even multiply palmitoylated peptides can be reliably analyzed by MS, though their hydrophobic nature poses challenges. Optimizing solvent systems and ionization conditions, such as incorporating DMSO, can enhance detection, paving the way for more comprehensive analysis of lipidated proteins in proteomics.
- Impact of Multiple S-Palmitoylation on Peptide Ionization and Fragmentation in Mass Spectrometry
Elisa Badin, Carla Schmidt
Chemistry-Methods 2025
https://doi.org/10.1002/cmtd.202500106