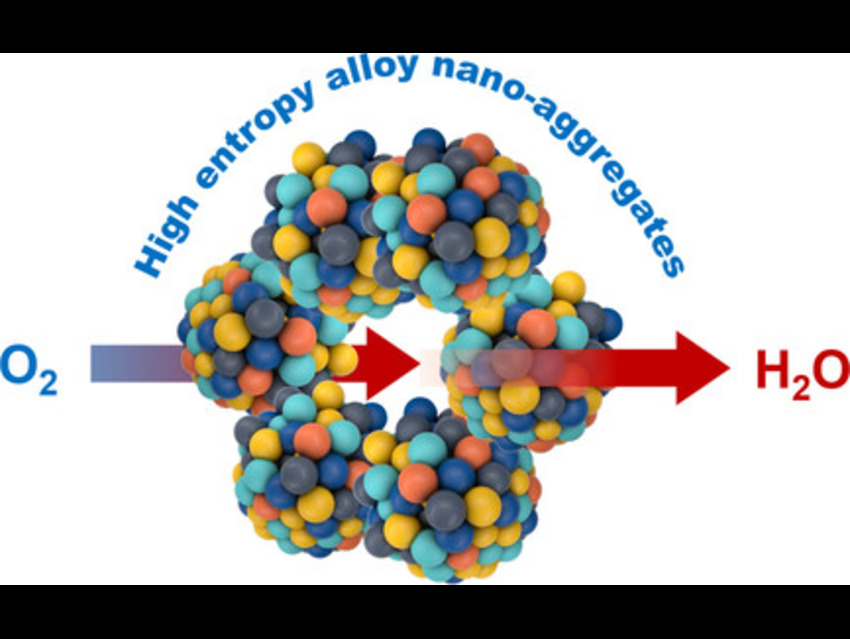
Fuel cells and metal–air batteries need catalysts that can efficiently reduce oxygen. Conventional platinum on carbon (Pt/C) catalysts are active but suffer from poor durability and methanol poisoning. Guangxing Yang and Feng Peng from Guangzhou University, China, and colleagues have designed a more stable and efficient alternative.
The team created PtRhPdIrRu high-entropy alloy (HEA) nanoaggregates by controlling particle dispersion through zeta potential. Noble metal precursors were dissolved in methanol, mixed with hydrochloric acid (HCl), and combined with carbon black. Droplets of this suspension were injected into hot triethylene glycol (TEG) at 230 °C. Rapid evaporation and coreduction produced nanoparticles that aggregated due to reduced zeta potential under acidic conditions. Lowering the pH protonated surface groups, reducing electrostatic repulsion and allowing van der Waals forces to drive aggregation.
Transmission electron microscopy (TEM) revealed particle sizes of approximately 4.2 nm, while X-ray photoelectron spectroscopy (XPS) confirmed metallic states with binding energy shifts indicative of electronic interactions among the constituent elements. Inductively coupled plasma mass spectrometry (ICP‑MS) and XPS showed near‑equimolar composition, and X-ray diffraction (XRD) confirmed a single-phase face-centred cubic (FCC) solid solution. Electrochemical testing demonstrated that HEA nanoaggregates showed superior oxygen reduction reaction (ORR) performance in both acidic (HClO₄) and alkaline (KOH) electrolytes, with higher half-wave potentials, greater mass activities, and lower Tafel slopes compared to commercial Pt/C. Rotating ring-disk electrode (RRDE) measurements confirmed a dominant four-electron pathway with reduced hydrogen peroxide formation.
Durability tests showed remarkable stability, with half-wave potentials (E₁/₂) of 0.851 V after 13,000 cycles in acid and 0.864 V after 30,000 cycles in base. Methanol tolerance experiments indicated strong resistance to poisoning.
Overall, the HEA nanoaggregates achieved up to 7.5 times higher mass activity than Pt/C while maintaining efficient four-electron ORR selectivity with minimal performance decay. These results demonstrate that HEA nanoaggregates outperform commercial Pt/C in both acidic and alkaline environments, combining high activity, durability, and methanol tolerance, offering promising pathways for more efficient fuel cells and metal–air batteries.
- High-Entropy Alloy Nano-Aggregates Enable Durable and High-Efficiency Oxygen Reduction Reaction
Jiahui Chen, Zenan Wu, Guoqiang Cao, Guangxing Yang, Qiao Zhang, Zhiting Liu, Feng Peng
ChemPlusChem 2025
https://doi.org/10.1002/cplu.202500489