Yuxiu Xiao, Long Yu, Wuhan University, China, and colleagues have developed a class of lanthanide-based metal–organic framework (Ln-MOF) nanozymes and uncovered a previously unknown affinity-driven catalytic mechanism that challenges the long-held “Lewis acidity–dominant” theory—the conventional belief that catalytic activity is primarily determined by metal ion Lewis acidity. Their findings establish a new paradigm for the rational design of nuclease nanozymes and open pathways for innovative biosensing technologies.
DNA, the fundamental carrier of genetic information, exhibits extraordinary stability under physiological conditions, with its phosphodiester backbone estimated to have a half-life of over 130,000 years. Natural nucleases can catalyze precise DNA cleavage but often lose activity under non-physiological or harsh conditions. Nanozymes, offering superior stability and tunability, have emerged as promising alternatives. Yet, despite intense research, their hydrolytic performance in DNA cleavage has remained disappointingly low due to an incomplete understanding of the catalytic mechanism.
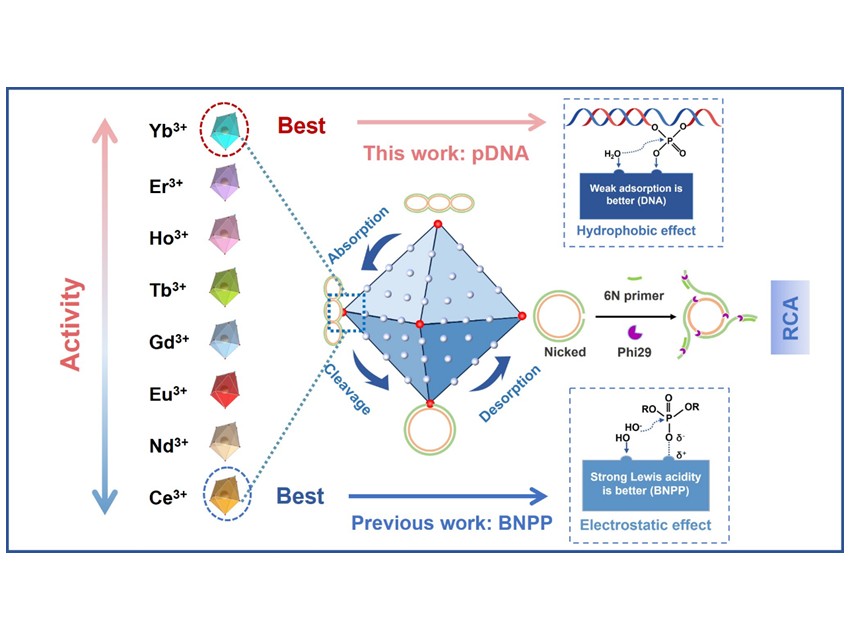
For decades, scientists assumed that activity was governed by Lewis acidity—the stronger the acid site, the higher the catalytic efficiency. However, this paradigm fails to explain a persistent contradiction: nanozymes highly active toward simple model substrates such as bis(4-nitrophenyl) phosphate (BNPP) often exhibit negligible activity toward real DNA.
To address this, the team systematically synthesized a series of Ln-BDC nanozymes (using eight lanthanides from Ce³⁺ to Yb³⁺ and terephthalic acid as the ligand) to isolate the influence of the metal center on nanozyme-DNA interactions. Their results overturned conventional wisdom: as the atomic number increased, catalytic activity toward DNA rose sharply, even though Lewis acidity weakened. Yb-BDC achieved a record DNA-cleaving half-life of approximately 30 minutes, marking the highest efficiency reported to date for artificial nucleases.
Mechanistic investigations revealed that catalytic performance is decoupled from acid strength and instead governed by a dynamic binding–release cycle between the nanozyme and DNA. Using isothermal titration calorimetry (ITC), the researchers demonstrated that weaker DNA affinity actually enhances catalytic turnover by facilitating product desorption and active-site regeneration—an “affinity-driven” mechanism distinct from traditional acidity-based models.
Building on this discovery, the team developed a CRISPR/Cas-inspired biosensing platform by integrating Yb-BDC with rolling circle amplification (RCA). The system successfully detected non-nucleic-acid biomarkers such as myoglobin (Myo) with ultra-high sensitivity, surpassing conventional enzyme-dependent assays.
This research not only resolves a long-standing mechanistic mystery but also establishes a new theoretical foundation for designing high-performance artificial nucleases and biosensors.
- Engineering Lanthanide Metal-Organic Framework Nuclease Nanozymes: Unveiling Affinity-Driven DNA Hydrolysis
Zhiwen Gan, Long Yu, Yongzhen Liu, Yumin Feng, Jiyu Tong, Yuxiu Xiao
Aggregate 2025
https://doi.org/10.1002/agt2.70180