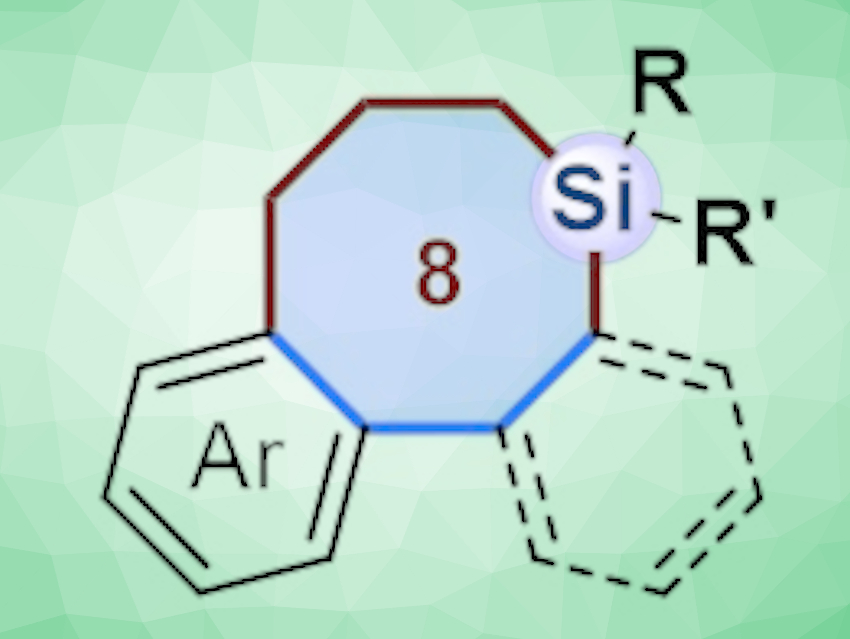
Incorporating silicon atoms into organic compounds can be useful in pharmaceutical chemistry to change the biological activity, lipophilicity, and toxicological properties of molecules. Biaryl compounds bridged by eight-membered rings are found in a variety of natural products. Thus, compounds in which silicon-containing eight-membered rings bridge biaryl units could be useful for finding new lead compounds in drug discovery. However, such compounds are challenging to synthesize.
Xiufang Xu, Dongbing Zhao, Nankai University, Tianjin, China, and colleagues have synthesized biaryls that are bridged by eight‐membered silacycles using a ring-expansion approach (pictured below). The team generated five-membered palladacycles, either via the C–C bond cleavage of biphenylenes or via the C–H bond activation of 2-iodobiphenyls or 1-(tert-butyl)-2-iodobenzenes. Then they obtained the desired silacycles via a formal cross-dimerization of the five-membered palladacycles with silacyclobutanes using a Pd(0)-based catalytic system. The catalyst system consists of Pd(dba)2 (dba = dibenzylideneacetone) and a 2-dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl (DavePhos) ligand.
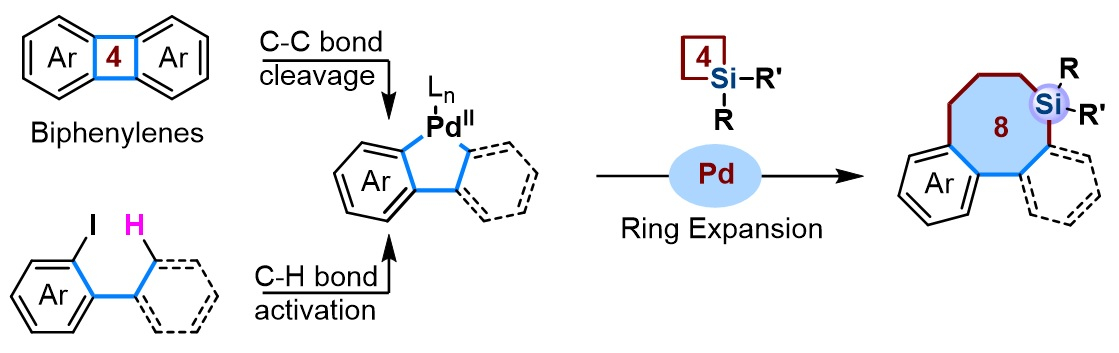
The approach provides access to structurally diverse biaryls bridged by eight‐membered silacycles, as well as corresponding benzoannulenes. Density functional theory (DFT) calculations indicate that the reaction of biphenylenes with silacyclobutanes involves a Pd0/II/IV cycle, initiated by oxidative C–C bond cleavage of biphenylenes.
- Ring Expansion to 8‐Membered Silacycles via Formal Cross‐Dimerization of 5‐Membered Palladacycles with Silacyclobutanes,
Xi-Chao Wang, Hao-Ran Wang, Xiufang Xu, Dongbing Zhao,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100535