“A black mouth is good for the stomach” is the motto the Haribo company used to advertise to customers at the end of the 1950s—and indeed, the root of the licorice plant is one of the oldest known remedies. In this article, we will take a deeper look at the chemistry of this healthy treat.
1 How Are Licorice Wheels Made?
1.1 Licorice
Licorice (Glycyrrhiza glabra) is an established remedy in all cultures. It was used in Chinese medicine as early as 2800 BCE, and is recommended in the Code of Hammurabi, Ebers Papyrus, the writings of Theophrastus and Pliny, and the books of Hildegard von Bingen for diseases of the stomach, as an expectorant cough medicine, and for many other maladies. Licorice remains a valued remedy today and has found its way into the German language in the expression “grating licorice”, which means to butter someone up with flattering compliments.
Licorice is a shrub that grows to about 1 m (see Fig. 1) in sandy soils of the temperate and subtropical areas of the Mediterranean, Middle East, Russia, and China. Licorice was cultivated in Bamberg, Germany, until the 19th century [1].
 |
|
Figure 1. Licorice (Glycyrrhiza glabra). |
1.2 Licorice Production
In the fall, the side roots of the plant are exposed, cut off, shredded, and extracted with boiling water. The glassy, black residue that remains after evaporation of the aqueous extract is called licorice block. To produce tasty licorice candy, the licorice block is boiled with water, flour, cane sugar, glucose syrup, plant oils, and flavoring and coloring agents. Then it is concentrated over 3–6 hours to a licorice mass with a water content of about 13–18 %.
In principle, cooking licorice just involves simple swelling and thickening of the flour, but the production of high-quality licorice is an art. In the cooking process, the flavor of top-quality raw product is enhanced with spices like ammonium chloride, which provides a salty/bitter flavor, and essential oils, especially aniseed oil [2]. In addition to the recipe, the preparation method (cooking temperature, cooking time, etc.) also affects the quality and character of the licorice. At over 80 %, the main ingredient in the essential oils from anise (Pimpinella anisum) and star anise (Illicium verum) is trans-anethole, or (E)-1-(4-methoxyphenyl)propene. Aniseed oil is used to give many alcoholic beverages their characteristic flavor: Pastis (e.g., Pernod), Raki, and Ouzo, herbal liquors (e.g., Benedictine, Boonekamp, Sonsdorfer), as well as Küstenebel and Goldwasser.
A RecipePrecise recipes and instructions for the production of successful licorice products are well-kept secrets. However, the following basic recipe [4] should be enough to satisfy a birthday party with about 1,000 kids: Combine 32 kg modified starch, 25 kg cane sugar, 31 kg glucose syrup, 10 kg glucose, 2 kg licorice block, and 28 kg water. Boil for several hours at 130 °C. Cool under vacuum to about 80 °C, pour into molds, and dry for about 40 hours at about 55 °C, until a water content of about 12 % is attained. |
1.3 Licorice Wheels
To make licorice wheels, the warm mass of soft licorice is pressed into cords, which are cut into 70 cm lengths and rolled into wheels. The finishing touch is a layer of beeswax and carnauba wax [3] to give the wheels their enticing shine. Carnauba wax is a wax of the leaves of the Brazilian carnauba palm, Copernica prunifera.
To those wishing to attempt making licorice wheels at home, it must be noted that stretching a cable with a uniform diameter of 3–4 mm out of a warm licorice mass is very difficult to pull off for a novice. The construction of a licorice wheel winding machine was a stroke of genius on the part of engineer Hans Riegel in the early 1950s. The 100 m-long monstrosity produced as many licorice wheels as 200 workers could have done. This mass production came at just the right time, because Germans devoured over 100 million licorice wheels a year in the 1970s, and the Haribo company became the market leader thanks to their licorice wheel winding machine [5]. The company name, Haribo, stands for Hans Riegel, Bonn.
2 The Chemical Secret
Like all natural products, block licorice is a complex mixture of countless chemical compounds; aside from water, there are about 11 % various saccharides, 28 % plant gum and starch, over 20 % pigments and other extracts, such as mineral salts. The fact that licorice does not just taste good, but also does good, is thanks to its 15 % content of glycyrrhizin (2) (see Fig. 2). This natural product has one singular property: it is 50 times sweeter than cane sugar.
2.1 70 Years of Confusion about the Molecular Formula of Glycyrrhizinic Acid
Several generations of researchers struggled to determine the chemical structure of glycyrrhizin. Initial experiments were reported by Vogel as early as 1843 [6]. From the lead salt, he was able to use hydrogen sulfide to obtain pure glycyrrhizinic acid C16H48O6. Three years later, Lade [7] split glycyrrhizin with nitric acid and was able to isolate a yellowish compound for which he proposed the formula C36H23O17.
In 1861, Gorup-Besanez [8] discovered an important structural feature of glycyrrhizinic acid: It is a glycoside, a sugar attached to a fragment known as an aglycone (“without sugar”). By boiling the glycyrrhizin with a mineral acid, the sugar could be split off, releasing the aglycone, which he suggested should be called glycyrrhetinic acid. He determined this compound should have a molecular formula of C36H52O18.
Further attempts to determine the structure failed and, growing frustrated, Gorup-Besanez ended his research in this area and termed glycyrrhizin an “ungrateful body.”
Glycyrrhizinic acid did turn out to be “ungrateful” because confusion over its molecular formula persisted for another 70 years: C44H64O19, Tschirch & Cederberg (1907); C45H72O6, Karrer [9,10] (1921); C23H36O3, Bergmann (1933); C30H46O4, Voss (1936); C30H46O4, Leuenberger and Ružička (1936). Without modern chromatographic separation techniques, the preparation of a pure substance from plants or animals was an extremely difficult and arduous undertaking. In fact, nearly 100 years passed between the first attempts to isolate glycyrrhizin and the preparation of larger amounts of the purified compound.
The degree to which Hans Leuenberger had to struggle as a doctoral student working with Leopold Ružička [11] in 1937 was recorded for posterity in his dissertation [12]. The image of the poor fellow waving a propeller back and forth over enormous ceramic dishes for days at a time is sure to raise some sympathy. Here is his description:
“5 kg shredded licorice plant are extracted two times with 25 L boiling water for 5 hours. After filtering off the plant material, the resulting solutions are combined and boiled. The sludge remaining in the extracts quickly masses together and can be removed after 24 hours. The 50 L of liquid are then concentrated to 4 L, which is best carried out in large ceramic dishes. If one finds some way to provide constant air movement over the surface of the liquid, for example, by blowing the vapors away with a propeller, the desired concentrate can be obtained relatively fast. The addition of 250 cc concentrated sulfuric acid causes the glycyrrhizin to precipitate out as tough, black clumps. Thorough kneading with water removes the sulfuric acid from the precipitate, which is then left to stand for 1 day. From time to time, the water that is slowly released from the bituminous cake is decanted off. The glycyrrhizin is dissolved by boiling two times in 1.8 L alcohol. The addition of potassium hydroxide results in potassium glycyrrhizinate as a tough brown mass. This is recrystallized from glacial acetic acid. The yield of very pure product is about 40–50 g.”
To obtain the aglycone, he then split the potassium salt of glycyrrhizin with concentrated hydrochloric acid. In the end, 5 kg of licorice plant yielded a meager 20–30 g of pure glycyrrhetinic acid.
2.2 Why Was it so Difficult to Isolate Glycyrrhizinic Acid?
Glycyrrhizinic acid (2) is found in licorice root as a mixture of the calcium and potassium salts (1) (see Fig. 2). 2 is a glycoside and consists of a sugar portion and an aglycone (“without sugar”) attached to each other through an acetal. The sugar component consists of two connected glucuronic acids and the aglycone is called glycyrrhetinic acid (3). Glycyrrhizin (2) can easily be split in an acidic medium to form a disaccharide and the aglycone glycyrrhetinic acid (3) (see Fig. 2).
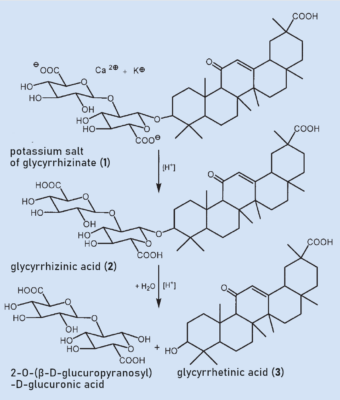 |
|
Figure 2. The active substance in licorice root is glycyrrhizinic acid (2), which is also known as glycyrrhizin. |
From our vantage point today, the difficulties of previous attempts at isolation are easy to explain. Depending on the method of preparation, the results included salt mixtures with varying amounts of different counterions (K+, Ca2+, NH4+, and Na+) and these “bituminous cakes” or “light brown, tough masses” were difficult to purify. Leuenberger was able to get a grip on the whole thing by immediately processing the difficult-to-purify substance (2) and its salts (1) to make glycyrrhetinic acid (3). Finally, with enough pure substance in hand, it was possible to begin finding its structure.
The first goal was to determine the molecular formula. From qualitative test reactions, it was known that glycyrrhetinic acid did not contain nitrogen or any other heteroatoms. It was made only of carbon, hydrogen, and oxygen. Finding the elemental composition would seem simple from this point: after combustion of a weighed amount of the unknown substance in excess oxygen, the amounts of the carbon dioxide and water formed were quantitatively determined. This combustion analysis method, introduced by von Liebig, gave the relative amounts of carbon and hydrogen. The oxygen could be calculated by subtraction.
Determining the elemental composition by percentages was not enough, however, because the compounds C5H7O2, C10H14O4, and C15H21O6 would give the same C, H, and O ratios on combustion. It was necessary to also determine the molecular weight through measurement of freezing-point depression or boiling-point elevation. The molecular formula could only be determined by combining the elemental composition with the molecular weight.
That is the theory, at any rate. In practice, the situation with glycyrretinic acid looked completely different: in the year 1937, there were two proposed molecular formulas (see Tab. 1): E. and F. Bergmann [13] had suggested C23H36O3 in 1933; however, teams led by Voss and Ružička [14] had proposed C30H46O4. Naturally, each team clung fast to “their” molecular formula. The Bergmann brothers were additionally displeased with Ružička, as he had not even cited their publication. In Helvetica Chimica Acta [15], they taunted, “It has clearly escaped their [Ružička’s and Leuenberger’s] attention that the same substances had already been … prepared several years ago,” before making their arguments against Ružička’s formula.
| Table 1. Molecular formula of glycyrretinic acid. | ||||||||||||||||||
|
The reply was swift [16]. Entitled “On the Empirical Formula of Glycyrrhetinic acid,” Ružička’s article tore his rivals apart: “The two authors [referring to the Bergmann brothers] continue to cling to this empirical formula, introducing certain useless arguments for it that we must discuss here. … we do not believe that this remarkable procedure of Bergmann’s … is allowed because it has obvious inherent flaws.”
Then the Bergmann brothers were additionally caught making a stupid computational error in calculating the molecular weight from X-ray photographic data: “It should also be noted that the numbers provided by Bergmann do not add up to give a molecular weight of 425, but rather 468.8, as reported to us by Dr. G. Giacomello, who checked the calculations. This is also in good agreement with the molecular weight for C30H46O4.”
Finally, Ružička struck his final blow: “In summary, it can be established that none of the objections raised by E. Bergmann and F. Bergmann against the C30H46O4 formula are valid.” For the Bergmann brothers, this ended in a technical knock-out: After their own control experiments, they were forced to concede that, “we have since successfully made a conclusive decision in favor of the formula proposed by Voss-Ružička.”
Although he was the clear victor, Ružička could not stop himself from firing off another salvo of logical acuity at the vanquished Bergmann brothers: “Bergmann describes [the results of his experiments] as ‘unambiguous evidence in favor of the Voss-Ružička formula.’ … These can obviously not be viewed as such evidence; it can only be concluded that these experiments provided new evidence against the C23 formula. The previously established evidence for the C30 formula had already been compiled by us.” No one can argue that chemists are not able to demonstrate ardor and passion—or to spew poison and bile!
2.3 And Today?
How would we determine the molecular formula for glycyrrhetinic acid?
2.3.1 Isolation of the Pure Substance
The first step would be to isolate the pure substance. In processing the licorice roots, we would have to follow in Leuenberger’s footsteps, although modern extraction equipment would make our lives much easier. We would also not need to wave a propeller in front of open ceramic bowls, because modern rotary evaporators can gently distill off large amounts of solvent in a short time. We can obtain crude glycyrrhetinic acid following Leuenberger’s procedure, and after a crude pre-purification, it can be purified by chromatographic processes. Since glycyrrhetinic acid is a major component of licorice, we would be able to obtain several grams of the pure compound within a few days by using modern high-performance liquid chromatography (HPLC) instruments.
2.3.2 Analysis
With the pure compound in hand, we can carry out standard combustion analysis to determine the relative elemental composition. However, mass spectrometry [17–19] is an unbeatable alternative because it can provide both the molecular weight and elemental composition.
In this method, the substance is vaporized under high vacuum and ionized by bombardment with electrons. The molecular ions are accelerated, and the application of electric and magnetic deflection fields makes it possible to determine the mass of the molecular ion, and thus, the molecular weight. However, it is not always so easy in practice, because the heaviest ion is not necessarily an intact molecular ion. Electron impact ionization is a truly brutal ionization process, and the initially formed molecular ion can sometimes fully decompose into lighter daughter ions that give the false impression of a smaller molecular weight. To confirm the result, additional measurements with gentler ionization methods, e.g., directly from a solvent matrix, must be used.
By using high-resolution mass spectrometry, it is possible to determine the molecular weight to several decimal places. This precision is sufficient to find the one correct combination of the isotopes 1H, 12C, and 16O among all the possible combinations: C30H46O4 (see Tab. 2).
By combining the exact atomic weights of the most common isotopes of the elements H, C, and O, it is possible to calculate the molecular formulas that best agree with the experimental result from the high-resolution mass spectrometry. For this calculation, it is necessary to add the atomic masses of the most common isotopes of each element (1H = 1.007825, 12C = 12.00000, and 16O = 15.994915) and not the chemical atomic weights that are based on the natural isotope distributions of the elements.
The molecular formula C23H36O3 (see Tab. 1) is ruled out from the start by the found molecular weight of 360. The precision of the high-resolution mass determination is under 0.005 mass units and is enough to unequivocally confirm a molecular formula of C30H46O4.
In addition, this molecular formula is the only one that agrees with the results of the elemental analysis (see Tab. 2, last column).
| Table 2. Mass spectrometry decides. | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
3 Licorice: Indulgence or Regret?
The curative effect of licorice root has been known since ancient times; licorice as a tasty candy has only been known since 1760, when clever pharmacist George Dunhill from Pontefract in England mixed licorice with sugar to make lozenges known as Pontefract Cakes.
Despite its popularity in folk medicine, clinical medicine did not take notice of licorice until 1955, after initial systematic studies of the successful treatment of gastric ulcers were reported. A short time later, glycyrrhetinic acid was identified as the main active ingredient (because glycyrrhizin-free licorice is also pharmacologically active, other ingredients, such as flavonoids and polyphenols, must also be active), and the succinic acid ester of glycyrrhetinic acid was successfully introduced into clinical practice under the name carbenoxolone. In addition to having an antibacterial effect against Helicobacter pylori that inhabit the mucosa of the intestine and duodenum—a major risk factor for ulcers in these areas [20], glycyrrhetinic acid is also effective against viruses and is used in HIV-1 patients to treat chronic liver infections.
Licorice preparations have also demonstrated numerous pharmacological effects (antiviral, interferon-stimulating, anti-inflammatory, radical scavenging, protective against cytotoxic damage, anti-carcinogenic) and have previously been and are currently being investigated in many clinical studies [21].
It can come as no surprise that overdoses of a pharmacologically active substance like licorice and its extracts could cause undesired side effects. In a New Zealand study [22], fourteen test subjects between 20 and 46 years of age were asked to eat 100 or 200 g of licorice every day. After some time, the first cases of edema (water accumulation) began to appear in the face and extremities. In some cases, these were so severe that the subjects left the study.
It was demonstrated in pharmacological studies [23] that glycyrrhetinic acid inhibits the enzyme 11-β-hydroxy-steroid dehydrogenase, which catalyzes the degradation of cortisol. This increases the retention time of cortisol in tissue. In cases of intestinal disease, this effect is certainly desirable, leading to faster healing of ulcers. The intake of extremely large amounts of licorice over an extended period leads to side effects that closely resemble a rare genetic disorder (only 18 cases described in the world) due to its interference in the body’s steroid management. These individuals have a deficiency in 11-β-hydroxy-steroid dehydrogenase and suffer from elevated blood pressure, low plasma potassium levels (hypokalemia), and resultant heart arrhythmia.
However, licorice lovers do not need to despair because—as is so often the case—everything depends on the right dose: In clinical cases, it is not about the consumption of licorice, but rather its abuse. Here are three curious examples [24]:
In a completely healthy 53-year-old man, the consumption of about 700 g of licorice daily for nine days led to the development of severe congestive heart failure, which was fully reversible upon cessation of licorice consumption.
A 38-year-old man was hospitalized due to heart arrhythmia. All therapeutic approaches were unsuccessful. Due to the declining condition of the patient, his laboratory values were checked again, revealing a low potassium level. Questioning of the patient revealed that he had eaten 400 g of licorice candies on every day of his hospitalization. After ceasing to eat licorice, his potassium level normalized, and his symptoms resolved.
This April, 48-year-old Margit K. of Berlin sued the Haribo company for 6,000 Euros in damages because she believed her heart arrhythmia was caused by the consumption of licorice candies. She claimed that the producer should have indicated this risk on the packaging. During the proceedings, it was revealed that she had consumed one 400 g bag of licorice candy every day for a period of several months. The lawsuit was dismissed on the basis that it is generally known that the consumption of sweets can lead to adverse health effects. This is true of nearly every food in the case of excessive consumption, and the producers do not need to provide a warning to this effect.
The German Federal Institute for Consumer Protection and Veterinary Medicine recommends [25] a maximum daily dose of 0.1 g glycyrrhizin. This limit, which corresponds to the daily consumption of roughly 50 g of licorice, should normally not be exceeded over a longer period of time, because a licorice lover savors licorice and does not subsist on it. As a precaution, the Federal Association of the Confectionery Industry has set a maximum content of 200 mg/100 g for licorice products produced in Germany [26]. Licorice products with a higher glycyrrhizin content are designated as strong licorice and can only be sold in pharmacies. But beware! This limit is only set on products in Germany. The amount of ammonium chloride (salmiak, E 510), which is approved as a flavor enhancer, is also limited to a 2 % content in Germany. This limit is also only valid for licorice products produced in Germany. Values up to 10 % have been measured in imported products.
4 Final Thoughts
When we slowly unwind a licorice wheel and appreciatively chow down on it, one centimeter at a time, we should reflect on how arduous the long journey from shredded licorice roots to the shiny, black wheel is and how much knowledge and technical skill it involves. The fact that licorice not only tastes good, but is also healthy when eaten in moderation, makes it even more desirable. As they appreciatively suck on their treats, licorice lovers who know their chemistry think about poor Hans Leuenberger, who had to boil down 50 L buckets full of licorice juice with a “propeller” for days at a time.
How do we get from the molecular formula C30H46O4 to the complicated structural formula (3) in Fig. 2? How did Leopold Ružička and his group, working practically alone for 30 years, with preparative skill, sharp wits, and endless patience, elucidate the structure as their crowning achievement? And how would chemists do it today?
We will report on this exciting aspect of the licorice wheel in the next part.
 |
|
Figure 3. Licorice in bloom (Photo: Pharaoh han, wikimedia commons, CC BY-SA 3.0). |
References
[1] J. G. Krünitz’s Ökonomisch-technologische Encyklopädie, Paulische Buchhandlung, Berlin, 1841.
[2] V. Koester, Anise Chemistry, ChemistryViews 2020. https://doi.org/10.1002/chemv.202000141
[3] K. Roth, Alle Jahre wieder: die Chemie der Weihnachtskerze, Chem. unserer Zeit, 2003, 37, 424–429. https://doi.org/10.1002/ciuz.200390086
[4] H. Hoffmann et al., Zucker und Zuckerwaren, Verlag Paul Parey, Berlin, 1985. ISBN: 9783489617143
[5] B. Grosse de Cosnac, Ein Bär geht um die Welt, Europa Verlag, Hamburg, 2003. ISBN: 9783203775210
[6] A. Vogel jun., Ueber den süssen Stoff aus der Süßholzwurzel (Glycyrrhizin), J. Prakt. Chem. 1843, 28, 1.
[7] T. Lade, Ueber Glycyrrhizin, Liebigs Ann. Chem. 1846, 59, 224. https://doi.org/10.1002/jlac.18460590208
[8] E. v. Gorup-Besanez, T. Klincksieck, Zur Kenntniss des Glycyrrhizins, Liebigs Ann. Chem. 1861, 118, 236–247. https://doi.org/10.1002/jlac.18611180213
[9] P. Karrer, W. Karrer, J. C. Chao, Glucoside VIII. Beitrag zur Kenntnis des Glycyrrhizins, Helv. Chim. Acta 1921, 4, 100–112. https://doi.org/10.1002/hlca.19210040108
[10] C. H. Eugster, Das Portrait: Paul Karrer 1889–1971, Chem. unserer Zeit 1972, 6, 147–153. https://doi.org/10.1002/ciuz.19720060503
[11] L. Ružička, Multimembered Rings, Higher Terpene Compounds and Male Sex Hormones (Nobel Lecture), 1939.
[12] H. Leuenberger, Zur Kenntnis der Glycyrrhetinsäure, Dissertation ETH Zürich, Switzerland, 1938.
[13] E. Bergmann, F. Bergmann, Biochem. Z. 1933, 267, 296.
[14] L. Ružička, H. Leuenberger, Polyterpene und Polyterpenoide CIX. Zur Kenntnis der Glycyrrhetinsäure, Helv. Chim. Acta 1936, 19, 1402–1406. https://doi.org/10.1002/hlca.193601901187
[15] E. Bergmann, F. Bergmann, Zur Kenntnis der Glycyrrhetinsäure, Helv. Chim. Acta 1937, 20, 207–208. https://doi.org/10.1002/hlca.19370200127
[16] L. Ružička, M. Furter, H. Leuenberger, Polyterpene und Polyterpenoide CXI. Über die Bruttoformel der Glycyrrhetinsäure, Helv. Chim. Acta 1937, 20, 312–325. https://doi.org/10.1002/hlca.19370200146
[17] W.-D. Lehmann, Physikalische Methoden in der Chemie: Massenspektrometrie. Vorstoß zu Makromolekülen I: Plasma-Desorption und Fast Atom Bombardment, Chem. unserer Zeit 1991, 25, 183–194. https://doi.org/10.1002/ciuz.19910250404
[18] W.-D. Lehmann, H.-R. Schulten, Physikalische Methoden in der Chemie: Massenspektrometrie II, Chem. unserer Zeit 1976, 10, 147, 163. https://doi.org/10.1002/ciuz.19760100602
[19] H. Budzikiewicz, Massenspektrometrie – Eine Einführung, Wiley-VCH, Weinheim, 1992. ISBN: 9783527210831
[20] R. Krausse, J. Bielenberg, Österreichische Apotheker-Zeitung, 2003.
[21] J. Bielenberg, Österreichische Apotheker-Zeitung, 2002.
[22] M. T. Epstein et al., Brit. Med. J. 1977, 1, 480.
[23] J. Bielenberg, Lakritzerzeugnisse – gesundheitlich unbedenklich?, Pharm. unserer Zeit 1992, 21, 157–158. https://doi.org/10.1002/pauz.19920210406
[24] R. Hänsel, K. Keller, H. Rimpler, G. Schneider (Hrsg.), Drogen, Springer, Berlin, 1998.
[25] BgVV rät zur Vorsicht beim Verzehr von Lakritze!, bfr.bund.de, 1999.
[26] R. Matissek, P.-D. Spröer, D. Werner, Bestimmung von Ammoniumchlorid in Lakritzerzeugnissen, Dtsch. Lebensm. Rundschau 2004, 100, 73.
The article has been published in German as:
- Die Lakritzschnecke,
Klaus Roth,
Chem. unserer Zeit 2004, 38, 202–207.
https://doi.org/10.1002/ciuz.200490046
and was translated by Caroll Pohl-Ferry.
The Licorice Wheel – Part 2
Let us accompany Leopold Ružička on his arduous journey from the molecular formula C30H46O4 to the structural formula
The Licorice Wheel – Part 3
How do chemists today determine the structure of a complex natural substance?
See similar articles by Klaus Roth published on ChemistryViews.org




