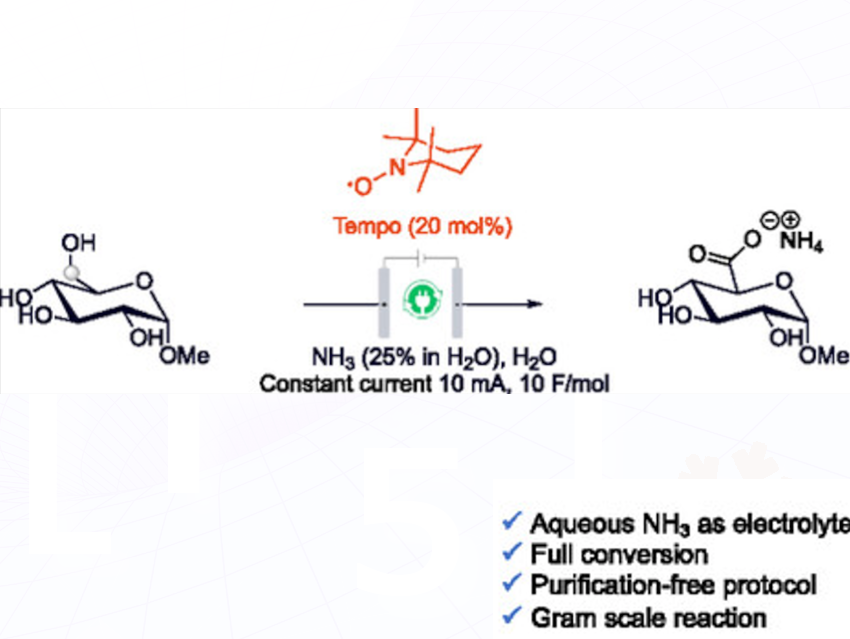
The study addresses the challenge of producing oxidized sugar molecules (uronic acids) in a more efficient and environmentally friendly way. Traditional approaches are often complicated, requiring harsh conditions and chromatography. To simplify this, Sebastian B. Beil, Max-Planck-Institute for Chemical Energy Conversion Stiftstr, Mühlheim, Germany, Adriaan Minnaard, Stratingh, University of Groningen, Nijenborgh, Netherlands, and colleagues developed a high-yielding electrochemical method that selectively oxidizes sugar derivatives at the C6 position. By using aqueous ammonia as both the electrolyte and the base, they achieved a cleaner, scalable process that eliminates the need for traditional purification steps.
The key method is TEMPO-mediated electrochemical oxidation done in water, using cheap and safe graphite or RVC (reticulated vitreous carbon) electrodes. A mild electric current was applied in an undivided cell, with aqueous ammonia serving two purposes: it increased conductivity and created basic conditions for the reaction. The reaction oxidizes the terminal primary alcohol group (C6) of sugar molecules like methyl-α-D-glucopyranoside into a carboxylic acid, forming ammonium uronate salts. By using lyophilization (freeze-drying) after the reaction, they could directly isolate the product without extra purification steps. The optimized conditions were extended to other sugar types, including glucosamine derivatives and galactopyranosides, achieving over 95% yield.
For less reactive sugars or those that form side products, modifications in mediator (4-acetamido TEMPO) or electrode type were successfully applied. A gram-scale synthesis was also demonstrated, showing the method’s practicality.
This method offers a clean, scalable, and efficient way to prepare sugar-derived acids without chromatography, which could be valuable in biomedical research, pharmaceuticals, and material sciences. It opens doors to more sustainable carbohydrate transformations and may inspire further development of electrochemical methods for biomolecule modification.
- A Purification-Free Strategy for the Electrochemical Oxidation of the Primary Hydroxy Group in Glycopyranosides
Marios Kidonakis, Ruben L. H. Andringa, Imke M. A. Bartels, Martin D. Witte, Sebastian B. Beil, Adriaan J. Minnaard
ChemElectroChem 2025
https://doi.org/10.1002/celc.202500140