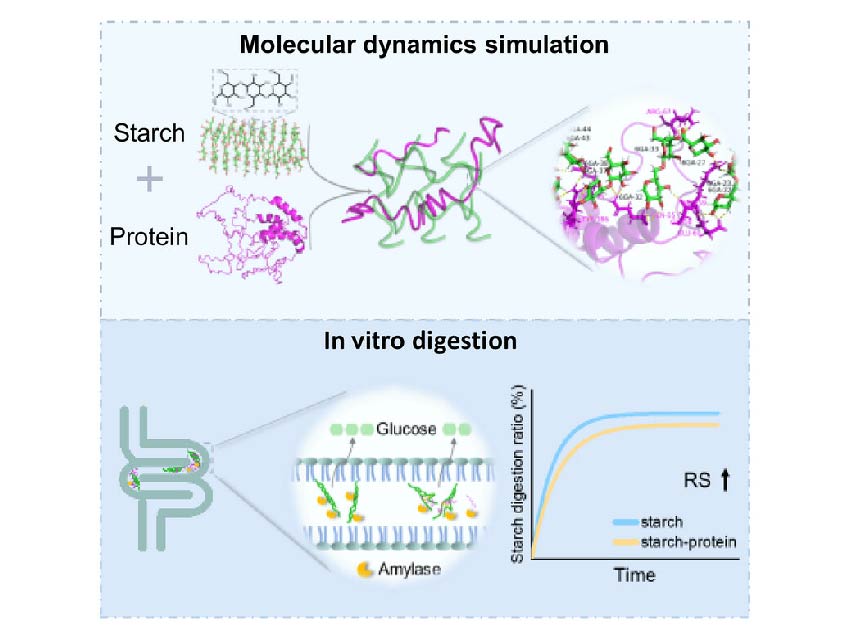
Combating diabetes requires starch-based foods with controlled digestibility, yet the molecular interactions between starch and proteins remain poorly understood.
Binjia Zhang, Southwest University, and Fengwei Xie, University of Nottingham Ningbo China (UNNC), along with their colleagues, discovered how wheat proteins (gliadin/glutelin versus globulin) form functional aggregates with starch that dramatically slow digestion.
Through integrated FTIR/XRD/rheology experiments and molecular dynamics simulations, the team revealed:
- Gliadin/glutelin bind starch 3× stronger than globulin (-107.67 vs. -99.50 vs. -35.21 kcal/mol)
- Hydrophobic and electrostatic interactions disrupt starch structure
- Enzyme inhibition increases resistant starch by 6.9%
- A novel competitive/non-competitive dual inhibition mechanism
The predictive framework links molecular interactions to digestibility, enabling precision design of low-glycemic foods—in other words, foods that release sugar more slowly, which could help manage diabetes.
- Molecular Aggregates of Wheat Starch–Protein Systems: Structural Disruption and Engineered Digestibility via Non-Covalent Synergy
Cuihong Dai, Dongling Qiao, Bowen Li, Fengwei Xie, Binjia Zhang
Aggregate 2025
https://doi.org/10.1002/agt2.70115