Pathogenic microorganisms, including drug-resistant bacteria and monkeypox virus, pose significant threats to human health and global public health security. While antibiotics remain clinical mainstays against bacterial infections, their overuse accelerates the evolution of untreatable superbugs.
By 2050, it is projected that 10 million people worldwide will die from drug-resistant infections each year. Simultaneously, the zoonotic monkeypox virus remains without approved targeted therapies, as repurposed smallpox antivirals carry unproven efficacy and safety profiles in large-scale applications.
These converging crises underscore the critical need for innovative therapeutic platforms capable of simultaneously addressing multidrug-resistant pathogens and enveloped viruses through non-antibiotic mechanisms, circumventing current limitations in conventional treatment paradigms.
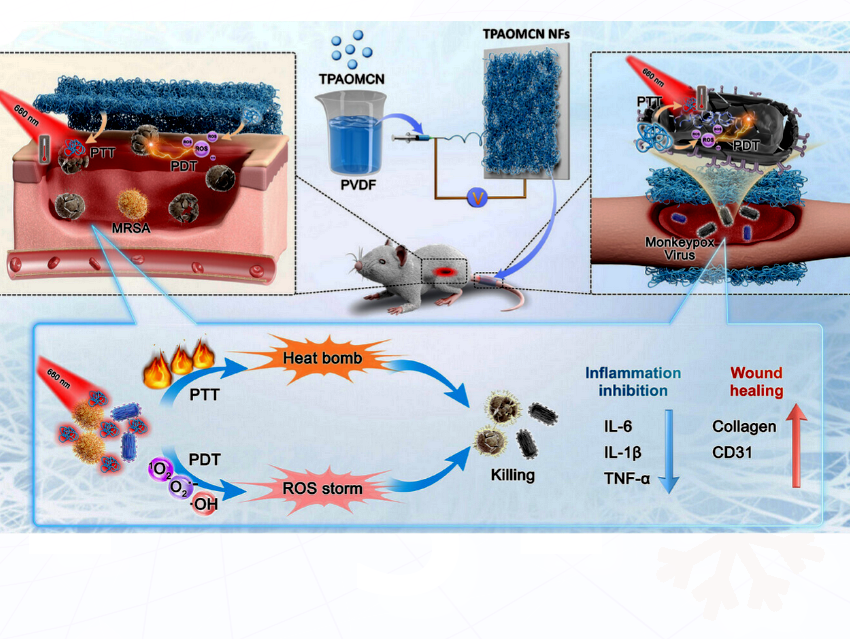
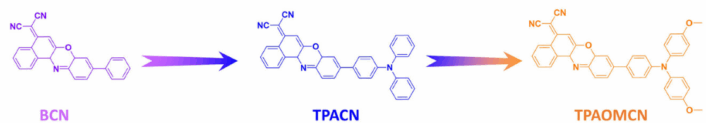
Gui Hua Jiang, The Affiliated Guangdong Second Provincial General Hospital of Jinan University, China, and colleagues designed and synthesized three Nile Red derivatives called BCN, TPACN, and TPAOMCN (pictured below) by a donor rotation and charge transfer enhancement strategy. This strategy combines molecular rotation and strong donor–acceptor interactions to maximize both heat generation and reactive oxygen species (ROS) production for dual phototherapeutic effects.
The team found that:
- By incorporating triphenylamine (TPA) with rich rotors, TPAOMCN showed effective non-radiative transition (NRT) and intense molecular rotation, achieving superior photothermal conversion capabilities.
- The strong intramolecular charge transfer of TPAOMCN made it less likely to release energy as light (weakened the radiative transition), which allowed it to more easily transfer energy to a special excited state. This smaller energy difference between the main excited state and the triplet state enabled the molecule to produce reactive oxygen species very efficiently, which are toxic to bacteria and viruses.
- In vitro experiments revealed that TPAOMCN NFs showed over 99.9% inhibition rate against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and MRSA (Methicillin-Resistant Staphylococcus aureus), as well as effectively eliminating the monkeypox virus.
- In both MRSA-infected wound and vaccinia virus-mediated tail scarification models, photoactivated TPAOMCN NFs exerted dual therapeutic-regenerative functions through pathogen eradication and inflammation modulation, achieving 90% accelerated wound healing in bacterial infections and 82% viral load reduction.
Overall, this study introduced TPAOMCN with exceptional photodynamic therapy (PDT), in which a light-activated compound produces reactive oxygen species that kill bacteria, viruses, or cancer cells, and photothermal therapy (PTT), where a light-absorbing compound converts light into heat to destroy harmful cells or pathogens. The study comprehensively demonstrated its safe and effective broad-spectrum activity against drug-resistant bacteria and viruses. These findings offered valuable insights and served as a reference for the development of next-generation clinical phototherapeutic materials against evolving microbial threats.
- Engineered Nile Red Derivatives-Mediated Efficient Phototherapy Against Drug-Resistant Bacteria and Monkeypox Virus
Laiping Fang, Wei Wang, Jianan Dai, Yike Tu, Shufang Li, Kuo He, Siya Tong, Yuhui Liao, Ping’an Ma, Guihua Jiang
Aggregate 2025
https://doi.org/10.1002/agt2.70130