Test your knowledge! Mass spectrometry is a powerful analytical technique that identifies and quantifies chemical species by measuring the mass-to-charge ratio of ions. It can be used with both magnetic sector and quadrupole systems.
This quiz below explores its fundamental principles and methods of characterization. And you can find a more advanced one on how mass spectrometry can detect proteins here.
 What is the fundamental principle behind spectrometry?
What is the fundamental principle behind spectrometry?
a) Measuring thermal conductivity of materials.
b) Investigating the interaction between matter and electromagnetic radiation.
c) Amplification of light signals for enhanced imaging resolution.
d) Testing electrical resistance.
Show the correct answer
Explanation: Spectrometry is based on how atoms and molecules interact with electromagnetic radiation. This includes absorption, emission, and sometimes scattering of light at specific frequencies, which correspond to unique energy transitions within the substance. These interactions produce spectral patterns that allow scientists to identify and quantify chemical species.
Unlike conductivity or resistance measurements, spectrometry focuses on optical properties, not electrical or thermal ones.
 Which property of atoms and molecules allows their identification using spectrometry?
Which property of atoms and molecules allows their identification using spectrometry?
a) Emitting sound at unique frequencies.
b) Absorbing or emitting electromagnetic radiation at specific frequencies.
c) Conducting electricity.
d) Their melting points.
Show the correct answer
Explanation: Spectrometry relies on the ability of atoms and molecules to absorb or emit electromagnetic radiation at specific frequencies, corresponding to unique energy level transitions. This interaction forms the basis of spectral identification.
 What is the primary purpose of mass spectrometry (MS)?
What is the primary purpose of mass spectrometry (MS)?
a) To measure the mass-to-charge ratio of ions.
b) To identify acoustic properties.
c) To calculate thermal expansion.
d) To determine the molecular mass and composition of chemical compounds.
Show the correct answer
Explanation: While mass spectrometry measures the mass-to-charge ratio of ions, its primary analytical purpose is to determine molecular mass and chemical composition, thereby enabling compound identification and structural analysis.
In mass spectrometry, molecules are first ionized using methods like MALDI or ESI.
- MALDI (Matrix-Assisted Laser Desorption/Ionization) is a method where molecules are mixed with a special chemical matrix and hit with a laser, which turns them into charged ions without breaking them apart.
- ESI (Electrospray Ionization) is a technique where a liquid sample is sprayed through a fine needle at high voltage, producing tiny charged droplets that release ions into the mass spectrometer.
These ions are then accelerated in an electric field and travel through a vacuum. In time-of-flight (TOF) instruments, lighter ions reach the detector faster than heavier ones, allowing measurement of their mass-to-charge ratio (m/z).
The separated ions (the initial molecule and/or its fragments) are detected, and their relative abundance is measured, producing a mass spectrum. This spectrum plots ion abundance against m/z values, providing a unique “fingerprint” for the compound. Atoms or molecules in the sample can be identified either by matching the measured ion masses to the exact masses of known molecules (for example, detecting the whole molecule as one ion) or by interpreting the unique fragmentation pattern, since different compounds break apart in characteristic ways that act like a structural signature.
 What distinguishes a magnetic sector mass spectrometer from other types?
What distinguishes a magnetic sector mass spectrometer from other types?
a) It uses light scattering for ion separation.
b) It filters ions using oscillating electric fields.
c) It separates ions using magnetic and electric deflection.
d) It relies on viscosity-based ion sorting.
e) It separates ions based on their differences in time of flight.
Show the correct answer
Explanation: Magnetic sector MS uses both magnetic and electric fields to deflect or redirect ions based on their mass-to-charge ratio, unlike quadrupole MS, which uses only electric fields.
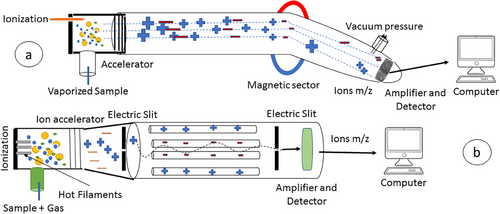
Schematic illustration of mass spectrometry by the magnetic sector (a) and quadrupole (b). Picture taken from [1]
 In a magnetic sector mass spectrometer, which component is responsible for accelerating the ions before separation?
In a magnetic sector mass spectrometer, which component is responsible for accelerating the ions before separation?
a) The detector.
b) The analyzer.
c) The electric field.
d) The sample holder.
Show the correct answer
Explanation: Before ions are separated by the magnetic field in a magnetic sector mass spectrometer, they are first accelerated by an electric field. This acceleration ensures that ions have sufficient kinetic energy for precise deflection based on their mass-to-charge (m/z) ratio.
Unlike magnetic sector MS, other types of mass spectrometers—such as quadrupole, time-of-flight (TOF), or ion trap—use different mechanisms for ion separation but still rely on initial acceleration by electric fields.
 Which component is universally present in all types of mass spectrometers?
Which component is universally present in all types of mass spectrometers?
a) Magnetic sector.
b) Quadrupole rods.
c) Ion source.
d) Laser emitter.
Show the correct answer
Explanation: All mass spectrometers require an ion source to generate ions from the sample, regardless of the analyzer type.
 What is the main function of the magnetic field in a magnetic sector MS?
What is the main function of the magnetic field in a magnetic sector MS?
a) To ionize the sample molecules.
b) To deflect/separate ions based on their mass-to-charge ratio.
c) To detect ions after separation.
d) To heat the sample.
Show the correct answer
Explanation: The magnetic field deflects ions in a curved path, with the degree of deflection depending on their m/z ratio. This allows for effective separation and analysis.
 How does a quadrupole mass spectrometer function to analyze ions?
How does a quadrupole mass spectrometer function to analyze ions?
a) By trapping ions in a magnetic field and measuring their spin.
b) By freezing ions and observing their color changes.
c) By separating ions based on their mass-to-charge ratio using oscillating electric fields.
d) By burning samples and measuring the resulting heat.
Show the correct answer
Explanation: A quadrupole mass spectrometer uses four parallel rods with oscillating electric fields to filter ions based on their mass-to-charge ratio (m/z). Only ions with a specific m/z can pass through to the detector, making it highly effective for targeted analysis in proteomics, metabolomics, and drug testing.
 Which of the following correctly matches mass spectrometry methods with their main characteristics?
Which of the following correctly matches mass spectrometry methods with their main characteristics?
a) MALDI-TOF: Uses a laser and a chemical matrix to ionize molecules; ideal for proteins, peptides, and microorganisms.
b) ESI MS: Produces ions from liquid samples; widely used for proteins, nucleic acids, and metabolites.
c) Quadrupole MS: Uses electric fields to filter ions by mass-to-charge ratio; common in quantitative analysis.
d) Ion Trap MS: Captures ions in a trap and sequentially ejects them for detection; useful for structural studies.
e) Magnetic-Sector MS: Uses magnetic fields to separate ions; historically important for high-precision measurements.
f) Orbitrap MS: Measures ion oscillation frequencies to determine mass with high resolution; used for proteomics and metabolomics.
g) Tandem MS (MS/MS): Involves two stages of mass analysis with fragmentation in between; enables detailed structural analysis.
Show the correct answer
Sources
Explore these resources to learn more about mass spectrometry:
 Mina Moradi, Zahra Farjami, Mohammad Mehdi Akbarin, Spectrometry and Its Application for the Detection of RNA-Binding Proteins: Advancements, Techniques and Challenges, Analytical Sciences Advances 2025. https://doi.org/10.1002/ansa.70026
Mina Moradi, Zahra Farjami, Mohammad Mehdi Akbarin, Spectrometry and Its Application for the Detection of RNA-Binding Proteins: Advancements, Techniques and Challenges, Analytical Sciences Advances 2025. https://doi.org/10.1002/ansa.70026- Igor V. Chernushevich, Alexander V. Loboda, Bruce A. Thomson, An introduction to quadrupole–time-of-flight mass spectrometry, Journal of Mass Spectrometry 2001. https://doi.org/10.1002/jms.207
 Chrys Wesdemiotis, Kayla N. Williams-Pavlantos, Addie R. Keating, Andrew S. McGee, Calum Bochenek, Mass spectrometry of polymers: A tutorial review, MassSpectrometryReviews 2023. https://doi.org/10.1002/mas.21844
Chrys Wesdemiotis, Kayla N. Williams-Pavlantos, Addie R. Keating, Andrew S. McGee, Calum Bochenek, Mass spectrometry of polymers: A tutorial review, MassSpectrometryReviews 2023. https://doi.org/10.1002/mas.21844- Ulrich Boesl, Time-of-flight mass spectrometry: Introduction to the basics, MassSpectrometryReviews 2016. https://doi.org/10.1002/mas.21520
Also of Interest