Compounds with unsupported metal–metal bonds are interesting research targets. However, unsupported f-block metal–metal bonded species, as well as compounds with unsupported f-block metal–main group metal bonds, are still rare. In particular, no Al–lanthanide-bonded species with an unsupported X-type Al ligand had been found so far.
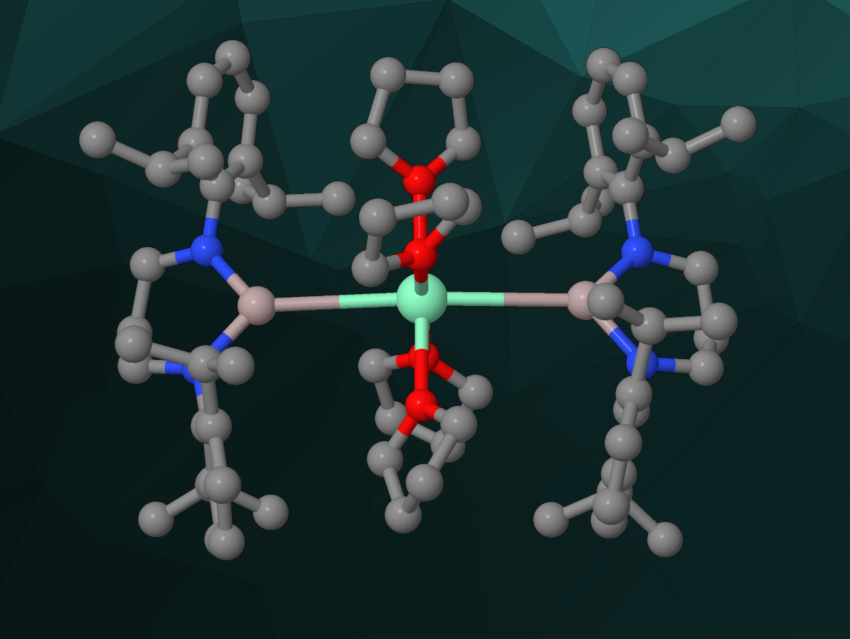
Zhenyang Lin, The Hong Kong University of Science and Technology, Kowloon, Makoto Yamashita, Nagoya University, Japan, and colleagues have synthesized a bis(alumanyl)samarium(II) complex with two unsupported Al–Sm bonds (simplified structure pictured). The team started from an alumanyl anion with a N,N‘-bis(2,6-diisopropylphenyl)-1,3-propanediamine ligand in a diethylether solution, which was reacted with SmI2(thf)2 in a tetrahydrofuran (THF) solution at –35 °C. Recrystallization then gave the desired bis(alumanyl)samarium complex in the form of pale green crystals.
The complex was characterized using single-crystal X-ray diffraction. The researchers found that it has a trimetallic Al–Sm–Al core with an almost linear arrangement and Al–Sm distances of ca. 3.518 Å and 3.543 Å. These distances are longer than previously reported Al–M bond lengths and suggest an ionic character. The team used density functional theory (DFT) calculation to better understand the bonding situation and found evidence for two highly polar Sm–Al σ-bonds.
The complex was reacted with carbodiimides, leading to a product in which four carbodiimides were inserted into the Al–Sm bonds of two bis(alumanyl)samarium complexes, and two more carbodiimides were coupled to form an oxalamidinate ligand that bridges the two Sm centers. A reaction with ethylene gave an Sm(II) complex with two anionic aluminacyclopropane rings. This species spontaneously isomerized to a complex with a dianionic 1,4-dialuminacyclohexane ring.
- Alumanyl-Samarium(II): Synthesis, Characterization, and Reactivity Studies,
Genfeng Feng, Ka Lok Chan, Zhenyang Lin, Makoto Yamashita,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c01193