Cyclotriveratrylenes (CTVs) can be used as chiral building blocks with dynamic planar chirality. They have a bowl-shaped structure that can racemize in solution via saddle-shaped conformations. When two CTV units are connected through spacers, a cage-like cryptophane is formed, and when one CTV is combined with another fragment in a similar manner, the resulting cage is called a hemicryptophane. While several conformations of CTV have been reported in caged structures, the precise control of its two bowl-like configurations has remained challenging.
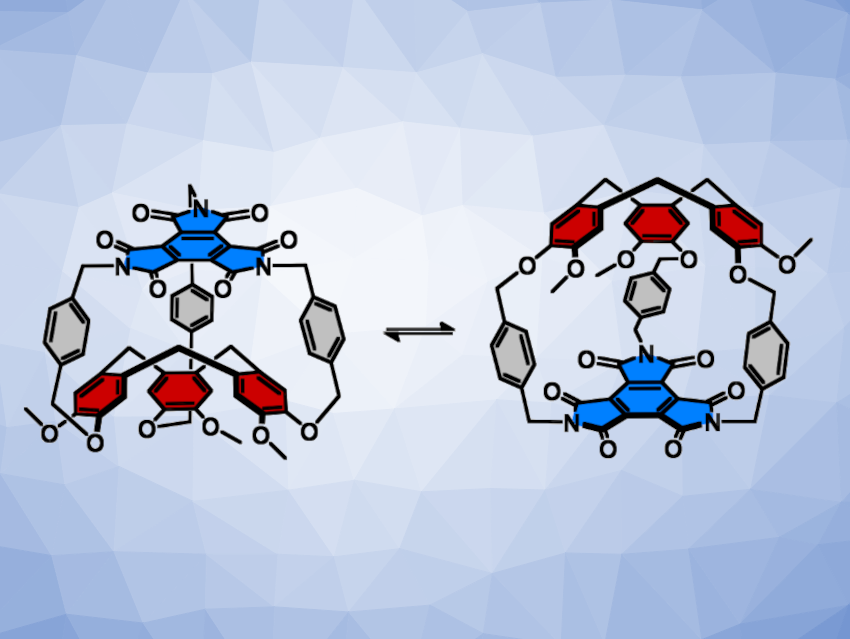
Alexandre Martinez, Yoann Cotelle, Aix-Marseille University, CNRS, France, and colleagues have developed a chiral molecular switch based on a CTV unit, in which the chirality inversion is controlled by the solvent (pictured below). The team built a hemicryptophane in three synthetic steps, starting with the nucleophilic addition of vanillyl alcohol to p-dibromoxylylene, followed by a triple Friedel-Crafts reaction and a reaction with benzenetriimide to close the cage.
The resulting product has a large cavity, which allows the CTV to switch between two configurations: Bulky aromatic solvents, such as 2-t-butylphenol, favor an “in” configuration with an “imploded” cage by desolvating the cavity (pictured below on the left). Smaller polar aprotic solvents, such as acetone, can enter the cavity and favor the “out” configuration (pictured below on the right). This can be used to reversibly switch the chirality of the cage.
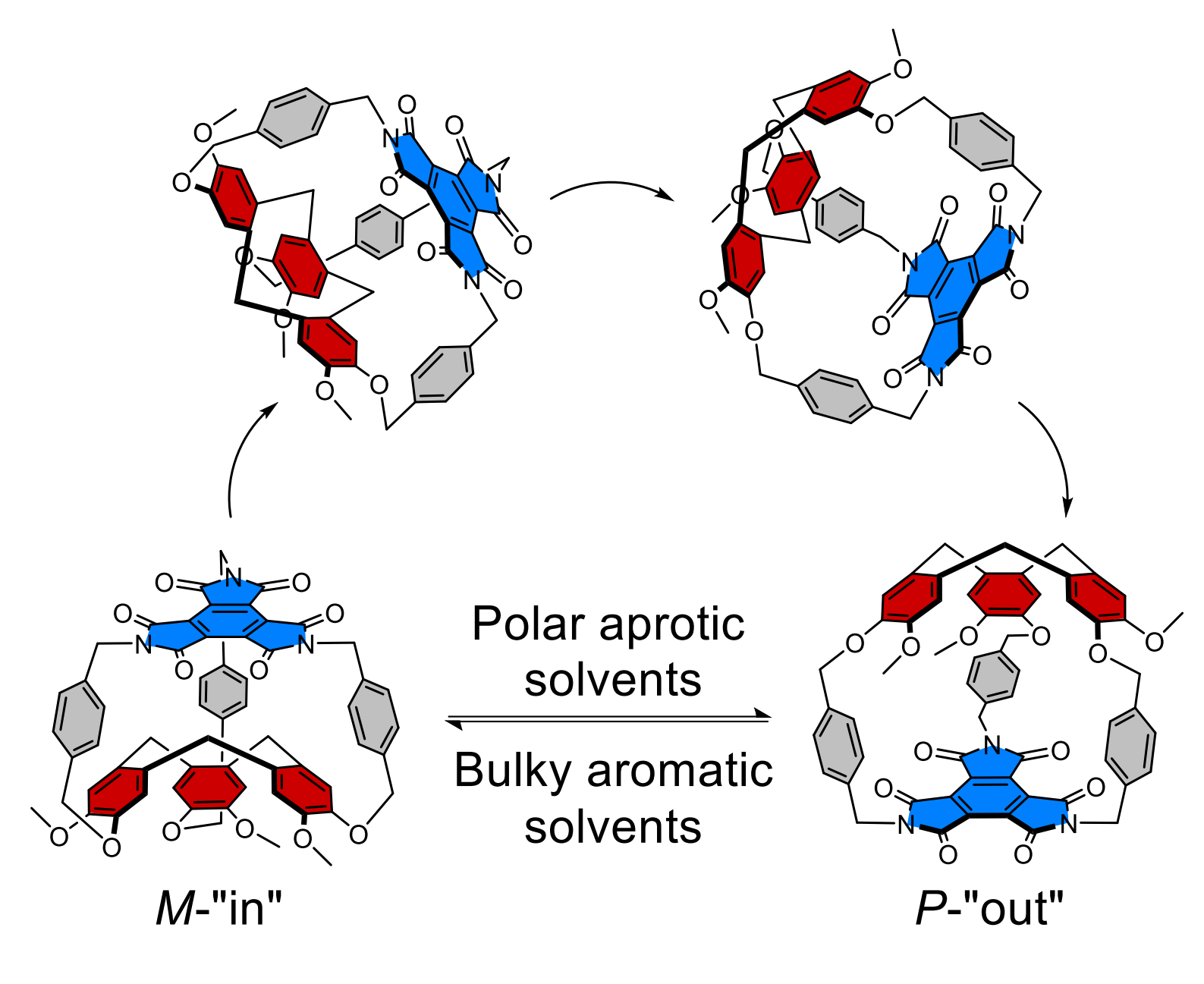
Overall, the work shows how solvents can induce drastic changes in the cavity of a molecular cage. It also demonstrates that CTVs could be useful for stimuli-responsive molecular machines.
- A Cyclotriveratrylene Solvent Dependent Chiral Switch,
Louise Miton, Élise Antonetti, Diego García-López, Paola Nava, Vincent Robert, Muriel Albalat, Nicolas Vanthuyne, Alexandre Martinez, Yoann Cotelle,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202303294