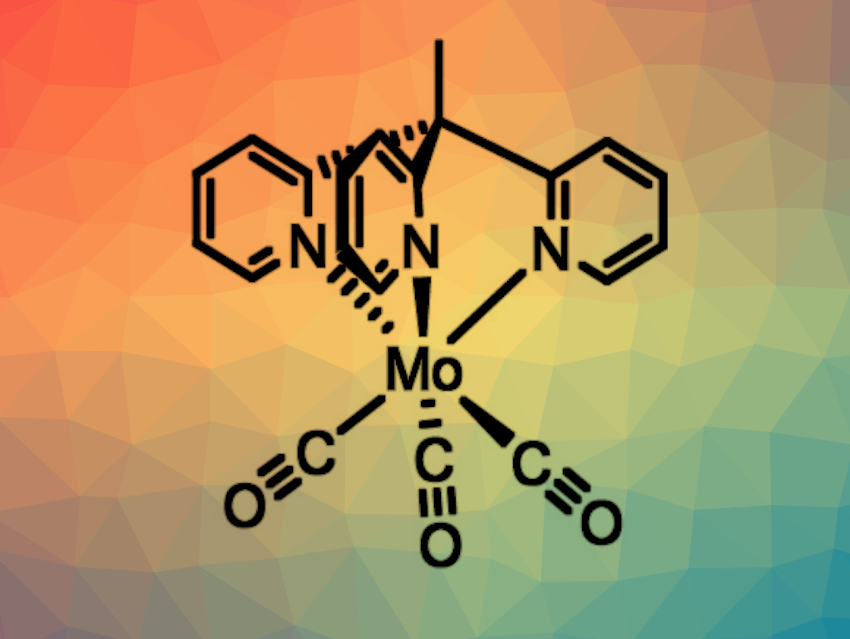
Metal complexes that are photoactive can have applications, e.g., in photocatalysis and light-emitting diodes (LEDs). Long-lived excited states are desirable in this context. However, when using earth-abundant metals, this often requires complex ligand scaffolds and laborious syntheses. Molybdenum(0) complexes, for example, could be promising, but they may require multi-step syntheses (as in the case of multidentate isonitrile complexes) or be hampered by limited stability (e.g., with carbonyl ligands).
Winald R. Kitzmann, Katja Heinze, University of Mainz, Germany, and colleagues have prepared a photoactive molybdenum(0) tricarbonyl complex (pictured) with a tripodal 1,1,1-tris(pyrid-2-yl)ethane (tpe) ligand in just two steps and found that the complex is very photostable. The team first synthesized the tpe ligand from commercially available 2-fluoropyridine and 2-ethylpyridine. The desired complex was then prepared by heating Mo(CO)6 and tpe in mesitylene/diglyme in a microwave for 10 min.
The complex exhibits intense deep-red luminescence with a lifetime of up to about 500 ns. It showed a higher photostability than a benchmark ruthenium complex under irradiation with green light. The team found that Mo(CO)3(tpe) can be used as a sensitizer in green-to-blue photon upconversion with 9,10-diphenylanthracene (DPA), providing a high upconversion quantum efficiency of 12 %. The complex can also serve as a photoredox catalyst in the dehalogenation of chloropyrazine. According to the team, the work might be useful for the further development of more sustainable photochemistry.
- Stable Molybdenum(0) Carbonyl Complex for Upconversion and Photoredox Catalysis,
Winald R. Kitzmann, Maria-Sophie Bertrams, Pit Boden, Alexander C. Fischer, René Klauer, Johannes Sutter, Robert Naumann, Christoph Förster, Gereon Niedner-Schatteburg, Nicolas H. Bings, Johannes Hunger, Christoph Kerzig, Katja Heinze,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c03832