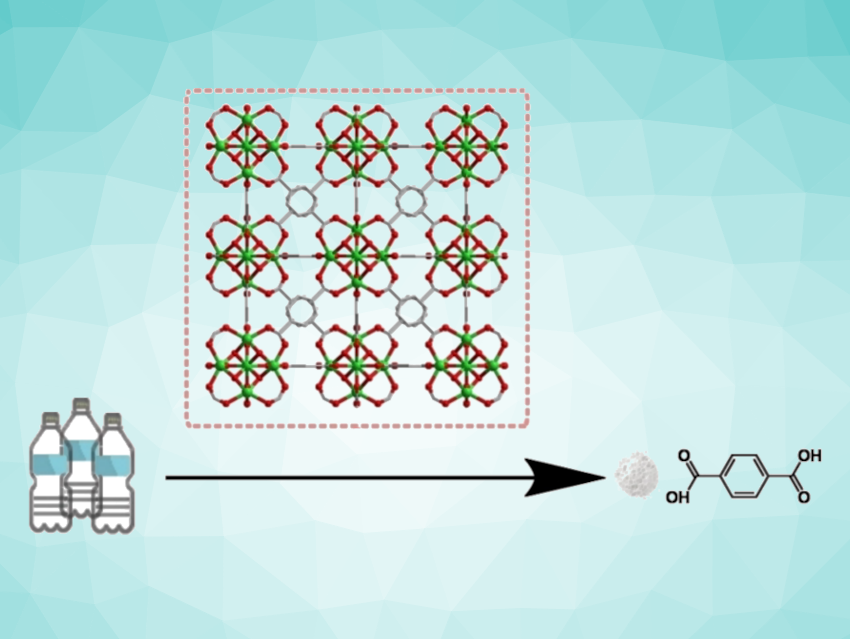
Polyethylene terephthalate (PET) is one of the most common plastics. Discarded PET most often ends up in landfills or in the environment because the rate of recycling is low. Omar K. Farha, Northwestern University, Evanston, IL, USA, and colleagues have found that a zirconium-based metal–organic framework material can catalyze the degradation of PET into its monomers. These can then be reused to make high-value PET, allowing the development of a circular economy.
PET Recycling
Almost 70 million tons of PET are produced annually. PET is processed to make products like fibers, drink bottles, and food packaging. Although PET can be melted down and reused, the high temperatures used for this result in a lower quality of the recycled products, limiting this strategy to only a few cycles.
The chemical deconstruction of PET into its monomers would provide a starting material for the renewed production of high-quality PET products. However, this requires large amounts of solvents and reagents, high pressures, and the expensive separation of troublesome byproducts. Additives and dyed products could also make things difficult. The alternative would be a catalytic process.
Catalytic Deconstruction Using a Metal–Organic Framework
The team found a catalyst that breaks PET waste down into the building blocks terephthalic acid (TA) and mono-methyl terephthalate (MMT) at 260 °C and in yields up to 98 %. The catalyst belongs to a class of materials known as metal–organic frameworks (MOFs), which are porous structures with metals at the nodes and organic molecules as linkers. The researchers chose to use UiO-66, a well-known zirconium-based MOF that can easily be produced on a large scale. UiO-66 consists of clusters containing six zirconium atoms as nodes, which are connected by terephthalic acid molecules as linkers.
Extensive structural analyses showed that during the deconstruction of PET, UiO-66 surprisingly rearranges into a different form with the same composition: MIL-140A, which is a framework of seven-coordinate zirconium oxide chains that are linked to six other chains by terephthalic acid bridges. This rearrangement only causes a slight decrease in the catalytic activity.
β-Scission or Hydrogenolysis
Detailed mechanistic studies indicate that the primary pathway for PET deconstruction is a β-scission. This reaction also plays an important role in thermolysis, but proceeds at a significantly lower temperature in the presence of the catalyst. Under hydrogen, the deconstruction also proceeds via hydrogenolysis. Polyethylene/polypropylene contaminants or dyed PET did not disrupt the deconstruction process.
These results illustrate the potential of established MOFs as a new class of polymer-deconstructing catalysts to overcome longstanding challenges related to plastic waste.
- Catalytic Degradation of Polyethylene Terephthalate Using a Phase‐Transitional Zirconium‐Based Metal–Organic Framework,
Yufang Wu, Xingjie Wang, Kent O. Kirlikovali, Xinyi Gong, Ahmet Atilgan, Kaikai Ma, Neil M. Schweitzer, Nathan C. Gianneschi, Zhong Li, Xuan Zhang, Omar K. Farha,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202117528


Exciting invention. I am fascinated by your work.