Protecting amino groups is often necessary in organic synthesis. Especially for complex syntheses, it can be useful to have different types of protecting groups available for one type of functional group. Amines can be converted to amides, which are usually very stable and, thus, challenging to use as protecting groups because the deprotection requires harsh conditions. Carbamates are often used instead because the deprotection can be driven by CO2 release in this case. However, they can be toxic and can complicate spectral data.
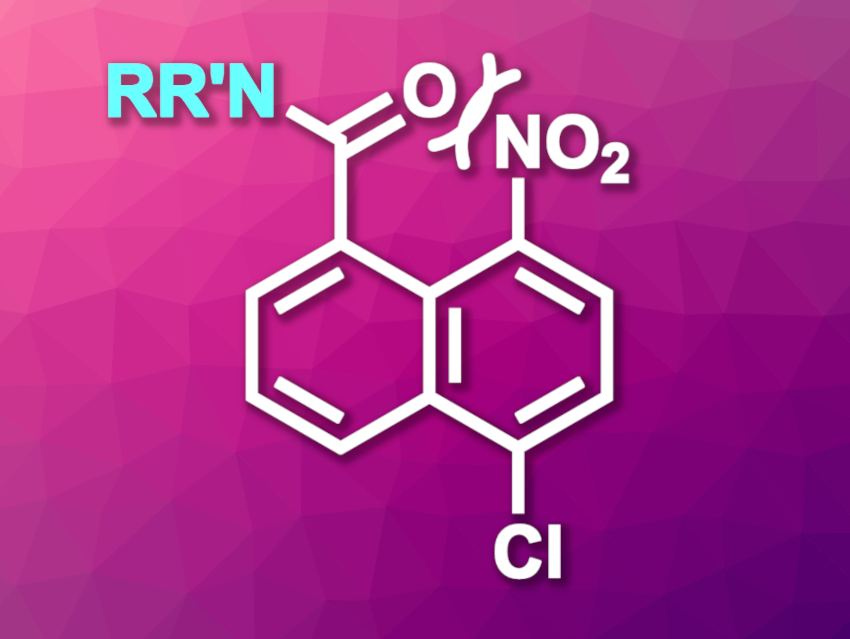
Victoria Alcázar, Polytechnic University of Madrid, Spain, Joaquín R. Morán, University of Salamanca, Spain, and colleagues have developed an alternative amide protecting group, i.e., 5-chloro-8-nitro-1-naphthoyl (NNap, general structure of the protected amine pictured above). With this group, there is a steric strain between the substituents at the 1- and 8-position of the naphthalene unit. The release of this steric strain allows deprotection under mild reductive conditions.
The team started from commercially available 1-naphthoic acid, which was brominated and nitrated. Treatment with thionyl chloride (SOCl2) then gave the acid chloride NNapCl. With the acid chloride, amines were then protected under Schotten-Baumann conditions in a two-phase reaction. To remove the protecting group, a suspension of Zn in acetic acid was used. During deprotection, the NNap group was converted to a lactam via an intramolecular cyclization. The developed protecting group could be a useful alternative for amine protection.
- 5-Chloro-8-nitro-1-naphthoyl (NNap): A Selective Protective Group for Amines and Amino Acids,
Asmaa Habib, José J. Garrido-González, Estela Sánchez-Santos, Irene Boya del Teso, Francisca Sanz, Victoria Alcázar, Ángel L. Fuentes de Arriba, Joaquín R. Morán,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01334