An air of decadence swirled around the preferred drink of Parisian Bohemians as the 19th century ended. Following decades of a complete ban, this high-ethanol beverage has been back on the market in the EU since 1991.
Originally, the green fairy kissed creative people after a couple of glasses, inspiring Baudelaire, Verlaine, Wilde, Toulouse-Lautrec, and van Gogh to create masterpieces while in its thrall. We would like to discover what chemical trick the “fée verte” used and find out whether there is still hope for a kiss from the green fairy when drinking modern absinthe—and how toxic it is.
4 The Effect of Thujone
A 1975 letter in Nature guided the attention of scientists back to thujone and absinthe. Because countless reports about the subjective effects of absinthe and marijuana (Cannabis sativa) seemed to be similar. Castillo et al. surmised that both active ingredients, thujone and tetrahydocannabinol (see Fig. 5), bind to the same receptor—the cannabinoid receptor—in the central nervous system [15]. This supposition later turned out to be false [16], but the anticipated legalization of absinthe rekindled interest in thujone. In their letter, the authors assumed that thujone binds to the cannabinoid receptor in its very energetically unfavorable enol form. This was a very bold hypothesis, to say the least. More recent research shows that thujone effects a different location in the nervous system.
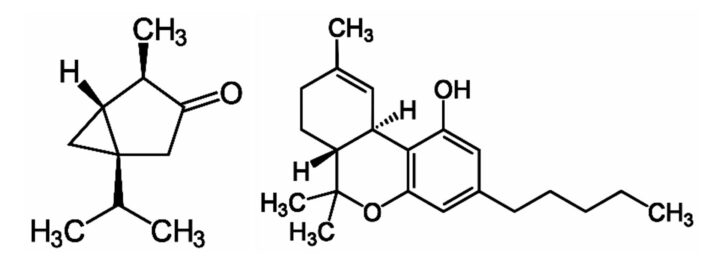
Figure 5. Thujone and tetrahydrocannabinol.
Grossly simplified, the activities of nerve cells are controlled by two chemical compounds (neurotransmitters). Stimulation is caused by glutamic acid, inhibition by GABA (γ-aminobutyric acid) [17]. GABA receptors are found in both the central and peripheral nervous system. Thujone blocks GABA receptors [18], preventing GABA from effectively inhibiting the nerve cells, making them extremely easy to stimulate.
The rapid continuous muscle cramps observed in animal experiments reflect this easy stimulation and are a consequence of the blocked GABA receptors.
4 Is Absinthe Damaging to Health?
Two questions are critical in judging the toxicology of absinthe: how toxic is thujone and how much of it is absorbed when drinking absinthe?
Although several studies were carried out with mixtures of thujone isomers of unknown composition, the published values for lethal doses in mice, rats, dogs, and guinea pigs are all in agreement at 70-400 mg/kg of body weight (see Tab. 1).
Table 1. Properties of thujone.
(As is so often the case, trivial names are confusing: there is a second substance known as isothujone, which is formed from the reaction of thujone with concentrated sulfuric acid. This constitutional isomer is formed by opening of the three-membered ring and is a cyclopentenone derivative.)
| Specific Rotation | Boiling Point | LD50 (mouse) | ||
|---|---|---|---|---|
| α-thujone (2) | (-)-thujone | -19.9 °C | Kp17 = 84 °C | 87.5 mg/kg |
| β-thujone (3) | (+)-isothujone [31] | +73.4 °C | Kp17 = 86 °C | 442.5 mg/kg |
The cramp-inducing dose (mouse, rat, rabbit, cat, not intravenous) of thujone is over 72 mg/kg [19], and rats demonstrated no changes in spontaneous activity or behavior after several weeks of daily intake of 10 mg/kg of thujone [20,21]. Based on current knowledge, it can be assumed that chronic thujone ingestion of 10 mg/kg of body weight is not damaging. If the green fairy does have a molecular formula of C10H16O, an absinthe drinker must have surpassed the 10 mg/kg limit, ingesting at least 700 mg of thujone. Only then can the fairy pay a visit.
The maximum amount of thujone absorbed can be estimated by using the original recipe (see Part 1). It calls for the use of 3.5 kg of wormwood to produce 100 L of absinthe. If the maximum oil content of the dried wormwood plant is 1.5 % and the thujone content of the oil is 80 %, a maximum of 400 mg of thujone can end up in one liter of absinthe. To surpass the limit of 10 mg/kg, a person would need to drink two liters of absinthe in a short amount of time.
However, after drinking a half liter at 74 Vol% alcohol (equivalent to one bottle of vodka), a visit from the green fairy might go completely unnoticed because the drinker would be completely intoxicated; a full liter of absinthe would raise the blood alcohol level above the lethal 5 % limit [22].
It must be emphasized that this is a worst-case calculation for determining the theoretical upper limit. Professional steam distillation delivers at most 0.2 – 0.4 % essential oil, and when other factors are taken into account, a realistic estimation [23] of the thujone levels are under 100 mg/L of absinthe. This means that the green fairy would only turn up for a visit after the consumption of seven (!) liters of absinthe.
In short, if the green fairy is hiding in thujone, a visit would go unnoticed, no matter how much absinthe we drink.
5 Was Absinthe Better in the Belle Epoque?
 A modern gas chromatographic analysis of an absinthe from 1900 (Pernod) and two absinthes produced using old recipes—one from an illegal distillery in Val de Travers, Switzerland, another from a modern distillery in Pontarlier, France—gave a surprising result: the old Pernod had the lowest thujone content and all three absinthes were significantly under the EU limit of 35 mg/L [24]. That old absinthes ever reached levels above 100 mg/L is highly doubtful.
A modern gas chromatographic analysis of an absinthe from 1900 (Pernod) and two absinthes produced using old recipes—one from an illegal distillery in Val de Travers, Switzerland, another from a modern distillery in Pontarlier, France—gave a surprising result: the old Pernod had the lowest thujone content and all three absinthes were significantly under the EU limit of 35 mg/L [24]. That old absinthes ever reached levels above 100 mg/L is highly doubtful.
When looking at the effects of old absinthes, other substances must also be considered. In the 19th century, absinthe was not always produced professionally. In fact, it was blended, watered down, and counterfeited. Inferior spirits containing far too much methanol and fusel alcohol were sometimes used—the bitter taste of wormwood disguised everything! The frequently observed vision problems can likely be attributed to the methanol.
Bad absinthe was “improved” by questionable methods: the green color was intensified with toxic copper sulfate, and inferior absinthe was commonly called “sulfat de cuivre” (copper sulfate); absinthe with added zinc sulfate was known as “sulfat de zinc”. The characteristic clouding (louche effect) was amplified by the addition antimony trichloride, which is hydrolyzed to form insoluble, white, basic antimony chlorides when water is added. It is impossible to know how many people at the time suffered physical and mental damage due to blending and additives.
6 A Glass à Votre Santé?
Today, comprehensive legislation and associated authorities provide for the definition and maintenance of quality standards in our foods. This is also true of absinthe, as the upper limit of 35 mg thujone/L set for high-alcohol-content bitter spirits by the EU is very much on the safe side, according to experts. The German Federal Institute for Consumer Health Protection and Veterinary Medicine (BgVV) determined that it is “not very likely that someone would ingest a dangerous amount of thujone by consuming absinthe” [25].
In their study, the BgVV investigated 30 absinthes available on the market and only one had a thujone content above the allowed limit at 45 mg/L. On the other hand, 25 of the absinthes had a thujone content below 10 mg/L. For twelve of the absinthes, it is doubtful that any wormwood was used in their production, because the thujone value was under 1 mg/L. Unfortunately, it is not a legal requirement that absinthe must be produced from wormwood, so herbal spirits produced entirely without it can be sold as absinthe.
With the official blessing of the BgVV, we can treat ourselves to a green hour with no worries. But will it taste good?
Not to worry: the staff at the BgVV did a thorough job in this area as well. In a truly heroic effort, they set their own health aside and tasted and evaluated 30 absinthes. They found a broad spectrum of taste and smell notes and aberrations: absinthe #12 smelled like old rubber and tasted raw, absinthe #16 smelled and tasted like pickle brine, and # 24 was slightly musty but did have a distinct herbal note. In other words, for true enjoyment, a good absinthe is required. Unfortunately, this is expensive, costing at least 30 EUR.
7 The Bitterness of Absinthe and Vermouths
Absinthe and vermouths (Martini, Cinzano & Co.) have a pleasantly bitter taste. The wormwood component that is responsible for this taste is absinthin, whose structure was determined in 1960 [14] (see Fig. 6).
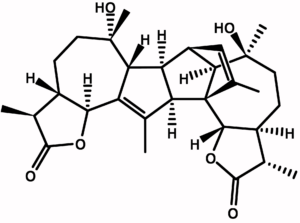
Figure 6. Absinthin, a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood).
Absinthin is non-volatile and does not transfer over with thujone during distillation; it is added during coloration. In this step, wormwood is directly extracted with the distillate and absinthin is taken up. Vermouth gets its bitter taste in the same way—the plant is directly extracted with the wine.
Wormwood is one of the most bitter plants and absinthin can be tasted even when diluted to 1:70,000. When drinking even the tiniest amounts, our innate distaste for bitterness raises the alarm—a fact that was used in folk medicine to help wean infants.
8 Conclusion
The question of where the green fairy is hidden in absinthe cannot be answered conclusively. We can exclude thujone because the amounts consumed are far too small. Was it maybe the large amount of alcohol? Was the green fairy a precursor to delirium? Or was it all just a delusion?
These are questions to be pondered over one, or at most two, glasses of absinthe.
As the sugar water slowly drips into the absinthe, as the green slowly grows milky, the fog lifts from our imagination and we can dream of a visit from the green fairy. Probably—no, certainly—we wait in vain, but what is the harm? Dreaming of the embrace of a beautiful fairy and a little, bicyclic ketone—with a three-membered ring, no less—is there a better way to while away the time?
Acknowledgements
I thank the following colleagues for their invaluable help with research and critical commentary: Dr. M.-C. Delahaye, Auvers-sur-Oise, France; Dr. J. Emmerich, Basel, Switzerland; M. Günther, Professor H. Hartl und H. Kleinhuber, Berlin, Germany; E.Vaupel, The Deutsches Museum Munich, Germany, and H. Werner, Hohenmölsen, Germany.
References
[15] J. Del Castillo, M. Anderson, G. Rubottom, Marijuana, absinthe and the central nervous system, Nature 1975, 253, 365–366. https://doi.org/10.1038/253365a0
[16] J. P. Meschler, A. C. Howlett, Thujone Exhibits Low Affinity for Cannabinoid Receptors But Fails to Evoke Cannabimimetic Responses, Pharm. Biochem. Behaviour 1999, 62, 473. https://doi.org/10.1016/S0091-3057(98)00195-6
[17] Povl Krogsgaard-Larsen, Bente Frølund, Tommy Liljefors, Specific GABAA agonists and partial agonists, The Chemical Record 2002, 2, 419. https://doi.org/10.1002/tcr.10040
[18] Karin M. Höld, Nilantha S. Sirisoma, Tomoko Ikeda, Toshio Narahashi, John E. Casida, α-Thujone (the active component of absinthe): γ-Aminobutyric acid type A receptor modulation and metabolic detoxification, Proc. Nat. Acad. Sciences USA 2000, 97, 3826. https://doi.org/10.1073/pnas.070042397
[19] Annette Erb, Die Grüne Fee: Thujon, Seminararbeit AG P. Schreier, Bayerische Julius-Maximilians-Universität Würzburg, Germany, 2003.
[20] B. H. Gahwiler, Organotypic cultures of neural tissue, Trends. Neurosci. 1988, 11(11), 484-489. https://doi.org/10.1016/0166-2236(88)90007-0
[21] R. Margaria, Acute and sub-acute toxicity study on thujone, Unpublished report of the Istituto di Fisiologia, Università di Milano, Italy 1963. (cited from CoE Datasheet RD4.2/14-44, 1999).
[22] Wolfgang Huckenbeck, Absinth – ein neues Spielzeug der Spaßgesellschaft?, SeroNews IV, Institut für Rechtsmedizin, Universität Düsseldorf 2001.
[23] B. Max, This and that: cheap drinks and expensive drugs, Trends Pharmacol. Sci. 1990, 11, 56. https://doi.org/10.1016/0165-6147(90)90315-y
[24] I. Hutton, Myth, reality and absinthe, Curr. Drug. Discov. 2002, 9, 62–64.
[25] M. Lang, C. Fauhl, R. Wittkowski, Belastungssituation von Absinth mit Thujon, BgVV Hefte Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin 08, Berlin, Germany, 2002. ISBN 3-931675-82-3
[30] Kenner C. Rice, Raymond S. Wilson, (-)-3-Isothujone, a small nonnitrogenous molecule with antinociceptive activity in mice, J. Med. Chem. 1976, 19(8), 1054–1057. https://doi.org/10.1021/jm00230a015
The Author
Professor Klaus Roth of the Free University of Berlin, Germany
The article has been published in German as:
- Der Zauber der Grünen Fee,
Klaus Roth,
Chem. unserer Zeit 2005, 39(2), 130–136.
https://doi.org/10.1002/ciuz.200590010
and was translated by Caroll Pohl-Ferry.
Absinthe – The Magic of the Green Fairy – Part 1
When drinking modern absinthe, is there still a chance of getting a kiss from the Green Fairy like the Bohemian Parisians at the end of the 19th century?
Absinthe – The Magic of the Green Fairy – Part 2
How toxic is Thujon and how much of it is ingested when drinking Absinthe?
See similar articles by Klaus Roth published on ChemistryViews.org




