Acid-autocatalyzed reactions are interesting chemical systems. However, the self-activation can be difficult to tune. Usually, acid-autocatalyzed systems are triggered on demand, for example, by adding a controlled amount of acid or by irradiation with light when photoacid generators are used. This need for external control can be overcome using in-situ acid generators.
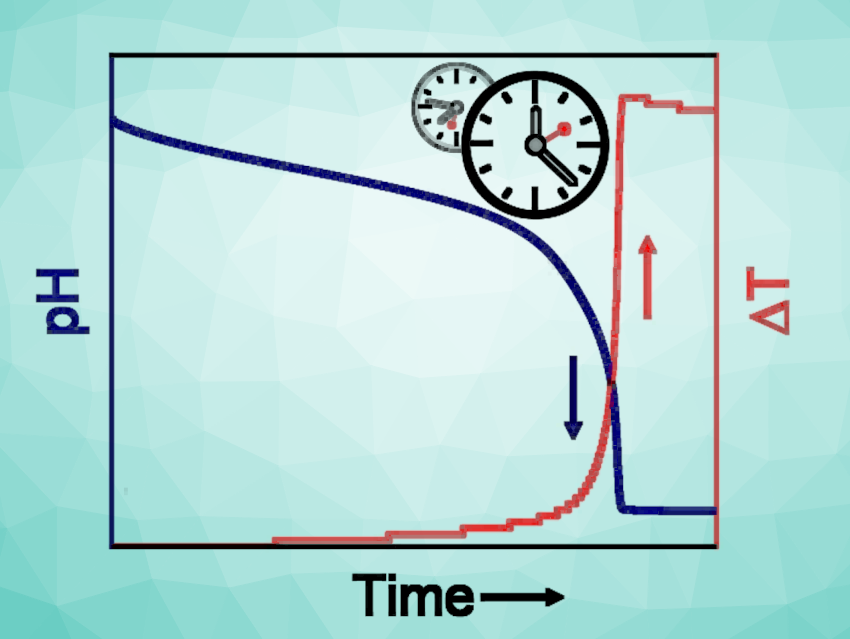
Clock reactions are chemical systems in which products appear sharply after an induction time—a behavior which often results in sudden chemical changes, e.g., of the pH. Ronny Kürsteiner and Guido Panzarasa, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, have developed a chemical system that can generate sudden temperature and pH changes after a tailorable induction time—a “thermochemical clock”.
The team found that it is possible to engineer an acid-autocatalyzed reaction network, the chlorate-sulfite-gluconolactone system (simplified mechanism pictured below), to generate a sudden temperature increase (up to ca. 30 °C) together with a pH change. The induction time and the magnitude of the temperature change can be tailored by carefully selecting the initial reaction parameters.
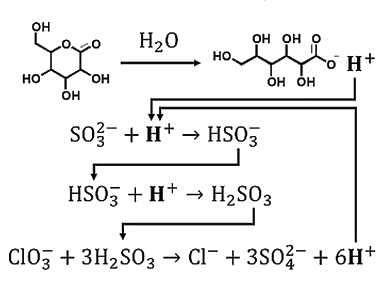
The work extends the variety of the stimuli that can be generated with chemical clocks. Such “thermochemical clocks” could be used, e.g., for controlling the self-assembly and actuation of temperature-responsive systems, from colloids to gels.
- Acid Autocatalysis Best Served Hot: The Chlorate–Sulfite–Gluconolactone System as a Thermochemical Clock,
Ronny Kürsteiner, Guido Panzarasa,
ChemSystemsChem 2023.
https://doi.org/10.1002/syst.202200042
(This article is part of a special collection on autocatalysis.)