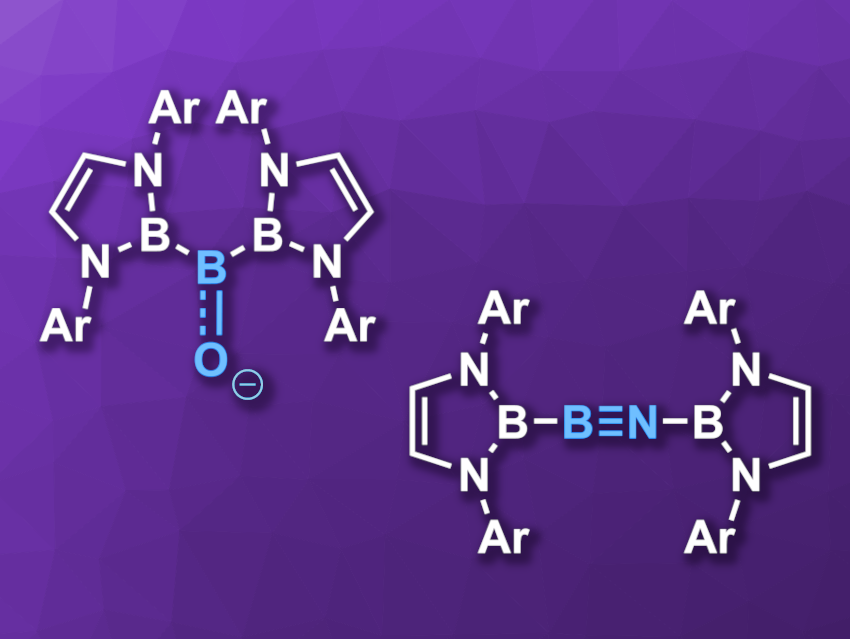
Oxoboranes (RB=O) and iminoboranes (RB≡NR) are comparable to the well-known ketones (R2C=O) and alkynes (RC≡CR), respectively. However, it is challenging to isolate monomeric oxoboranes because they tend to oligomerize due to the polarized B–O bond. Base- and acid-stabilized oxoboranes have been isolated, but obtaining acid/base-free examples is difficult. Iminoboranes can also be challenging targets for isolation due to their high reactivity.
Yuanting Suon, Soochow University, Suzhou, China, and Nanjing University, China, and colleagues have synthesized the first diboryl-substituted acid/base-free acyclic oxoborane and iminoborane compounds (pictured, Ar = 2,6-i-Pr2C6H3). They used bulky 1,3,2-diazaborolyl ligands to stabilize these species.
The team synthesized the acyclic oxoborane (pictured on the left) from the corresponding diboryl-substituted hydroxylborane via a reaction with potassium bis(trimethylsilyl)amide (K[N(SiMe3)2], KHMDS) in toluene at room temperature, using [2.2.2]cryptand to capture the potassium ion. The desired product was obtained in a yield of 90 %. The iminoborane was prepared from a diboryl-substituted chloroborane via a reaction with azidotrimethylsilane (Me3SiN3) in tetryhydrofuran (THF) in toluene at room temperature. The product was obtained in a yield of 62 %.
Both compounds were fully characterized. X-ray crystallography showed that the central boron atom in the oxoborane is coordinated in a trigonal planar manner and that the iminoborane has a nearly linear structure in the B–B–N–B unit. The results of density functional theory (DFT) calculations indicate that the compounds feature B=O and B≡N multiple bonds, respectively, both of which are more polarized and weaker than the corresponding bonds in comparable ketones/imines.
- Acid/Base-Free Acyclic Anionic Oxoborane and Iminoborane Bearing Diboryl Groups,
Manling Bao, Yuyang Dai, Chunmeng Liu, Yuanting Su,
Inorg. Chem. 2022.
https://doi.org/10.1021/acs.inorgchem.2c00966