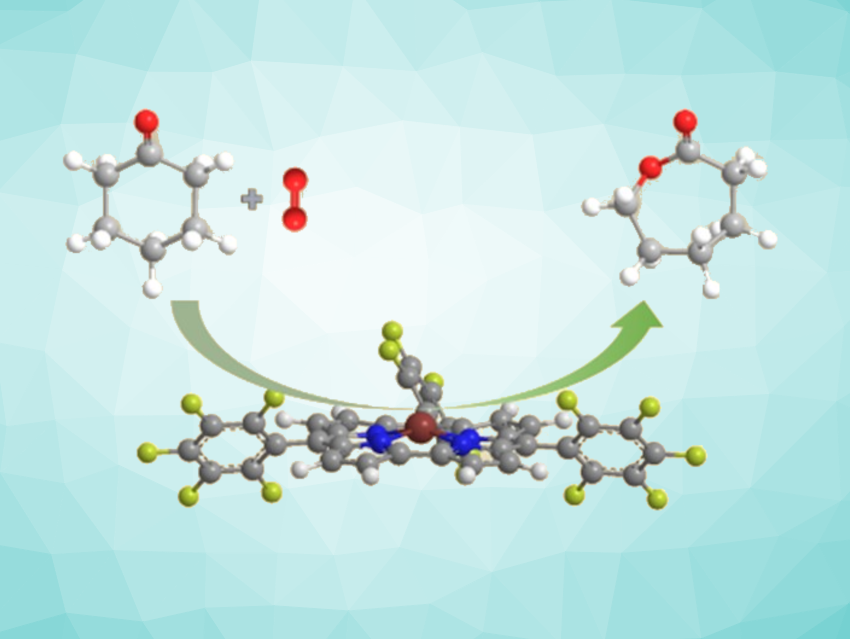
The Baeyer–Villiger oxidation is a classic transformation used to convert ketones into esters and cyclic ketones into the corresponding lactones. One example of an industrially important lactone is ε-caprolactone, a useful monomer for the synthesis of environmentally friendly degradable polymers. Improved lactone syntheses for industrial use could, thus, have economic benefits. Corroles are macrocycles that contain four pyrrole-type rings. Metal corroles can be effective catalysts in different types of transformations and have shown good activity and selectivity in catalytic oxidations, in particular.
Hai-Yang Liu, South China University of Technology, Guangzhou, China, and colleagues have developed the first metal-corrole catalyzed Baeyer–Villiger oxidation reaction, using the conversion of cyclohexanone to ε-caprolactone a model reaction (pictured). The team tested Co, Mn, Fe, and Cu complexes of 5,10,15-tris(pentafluorophenyl)corrole (tpfc)—namely, Fe(tpfc)Cl, Co(tpfc)PPh3, Mn(tpfc), and Cu(tpfc) as catalysts in the presence of benzaldehyde as a co-oxidant. The Cu complex had no catalytic activity, and the Mn and Co complexes showed poor catalytic performance. When Fe(tpfc)Cl was used as catalyst, up to 73 % yield were obtained in 6 h at a catalyst loading of only 0.1 mol%. 4Å-MS (molecular sieve) as an additive further improves the yield.
The researchers investigated the possible mechanism of the reaction. They used 2,6-di-tert-butyl-4-methylphenol as a free radical scavenger and found that the oxidation of cyclohexanone was completely inhibited. This suggests that free radicals play key roles in the reaction. The proposed catalytic mechanism starts with the auto-oxidation of benzaldehyde to peroxybenzoic acid via benzoyl radicals, initiated by Fe(tpfc)Cl. Then, peroxybenzoic acid oxidizes Fe(tpfc)Cl to produce an intermediate with an Fe(V)=O unit, which reacts with cyclohexanone to form a Criegee adduct and then releases the product.
- Aerobic Baeyer−Villiger oxidation catalyzed by metal corroles,
Meng-Ni Li, Bei Wan, Shuang Yang, Yan Tang, Hao Zhang, Si-Quan Zhang, Hai-Yang Liu, Yong Ye,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200462