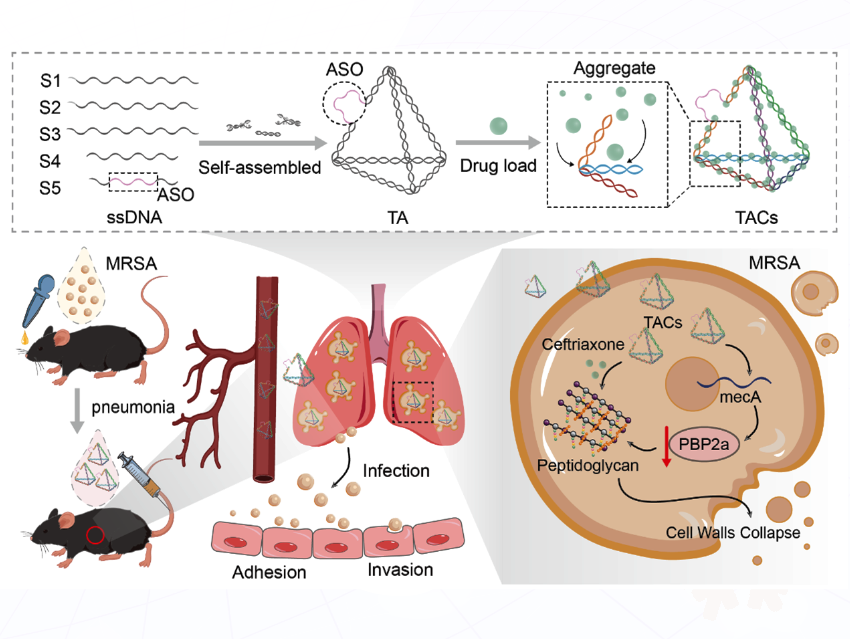
Changfeng Zhu from Fudan University, China, and colleagues have introduced a pioneering nanotherapeutic strategy against methicillin-resistant Staphylococcus aureus (MRSA). Centered on a “precision targeting with synergistic enhancement” paradigm, the team developed a multifunctional nanoplatform—TACs (tFNAs-ASOs-Ceftriaxone)—based on tetrahedral framework nucleic acids (tFNAs), offering a novel and highly effective approach for tackling multidrug-resistant bacterial infections.
This innovative system co-delivers antisense oligonucleotides (ASOs) targeting the mecA gene—a central determinant of MRSA β-lactam resistance—and the conventional antibiotic ceftriaxone within a self-assembling, aggregation-enhanced tFNA nanocarrier. By exploiting the spatial configuration and programmable functionality of tFNAs, TACs enables efficient drug loading, site-specific accumulation, and stimuli-responsive, sustained release at the site of infection. The dual-action mechanism orchestrates both genetic suppression of resistance and pharmacological amplification of antibiotic activity. In vitro antimicrobial testing revealed that TACs enhanced MRSA susceptibility to ceftriaxone by 16-fold, reducing the minimum inhibitory concentration (MIC) from 256 μg/mL to 16 μg/mL. Mechanistic investigations revealed that TACs penetrates MRSA cells with high efficiency, enabling targeted ASO-mediated knockdown of mecA expression, leading to substantial downregulation of PBP2a protein levels and restoration of β-lactam sensitivity. In vivo studies further validated the therapeutic potential of TACs. In a murine model of MRSA-induced pneumonia, TACs treatment significantly reduced pulmonary bacterial burden and inflammation while improving survival rates. Similar efficacy was observed in Galleria mellonella infection models, underscoring the broad-spectrum applicability of the platform in diverse and complex infectious contexts.
The TACs system represents a paradigm shift in the treatment of antimicrobial-resistant infections. Its advantages include multi-target synergy (simultaneous genetic and pharmacological disruption), intelligent nanocarrier-mediated delivery (enhancing drug localization and minimizing systemic toxicity), and platform versatility (compatibility with various antibiotics and gene targets). More than just a new drug delivery vehicle, TACs embodies a next-generation therapeutic approach—integrating gene silencing with conventional antibiotic therapy—offering a compelling solution in the global effort to overcome antimicrobial resistance.
- Multifunctional Framework Nucleic Acid Vehicle for Antibiotic Sensitization and Treatment of Methicillin-Resistant Staphylococcus aureus
Yicheng Zhao, Jingyi Si, Shisong Jing, Bingmei Wang, Yueshan Xu, Jiyu Guan, Quan Liu, Jianlei Shen, Min Lv, Li Wang, Changfeng Zhu
Aggregate 2025
https://doi.org/10.1002/agt2.70059