Molecular aggregation affects the interactions between molecules and can be a useful tool, e.g., in materials science. Its value for chemical reaction development has been far less explored. In solvent-free reactions, reactants are in close contact with each other, and such an aggregated state may benefit the reaction. Research attention has been paid to the sustainable features of solvent-free reactions, but their potential to promote reactions has not been well-studied.
Chen-Guo Feng, Shanghai University of Traditional Chinese Medicine, China, and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Chunsen Li, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Yingbin Liu, Shanghai Jiao Tong University School of Medicine, China, and colleagues have developed a method for aggregation-enabled alkene insertion into carbon–halogen bonds (pictured). The team reacted different aryl ethylenes with benzyl chloride, bromide, and iodide derivatives at 80–120 °C for 1–5 h under air. The corresponding insertion products were obtained in moderate to excellent yields.
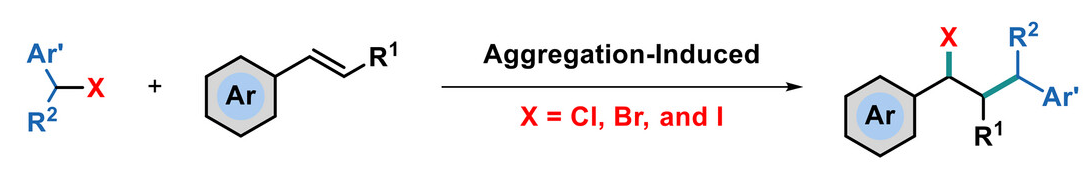
According to the researchers, the spontaneous cleavage of carbon–halogen bonds generates an intimate ion pair, which can be quickly captured by alkenes in the aggregated state. Additional catalysts or promoters are not necessary for the transformation. Experiments with added solvent indicate that the aggregated state is critical for the reaction. The method provides a broad substrate scope, high functional group tolerance, simple operation, high atom economy, and sustainability. The work could encourage further reaction development based on an aggregation strategy.
- Aggregation‐enabled alkene insertion into carbon–halogen bonds,
Meng‐Yao Li, Xiao‐Mei Nong, Han Xiao, Ao Gu, Shuyang Zhai, Jiatong Li, Ge Zhang, Ze‐Jian Xue, Yingbin Liu, Chunsen Li, Guo‐Qiang Lin, Chen‐Guo Feng,
Aggregate 2023.
https://doi.org/10.1002/agt2.346