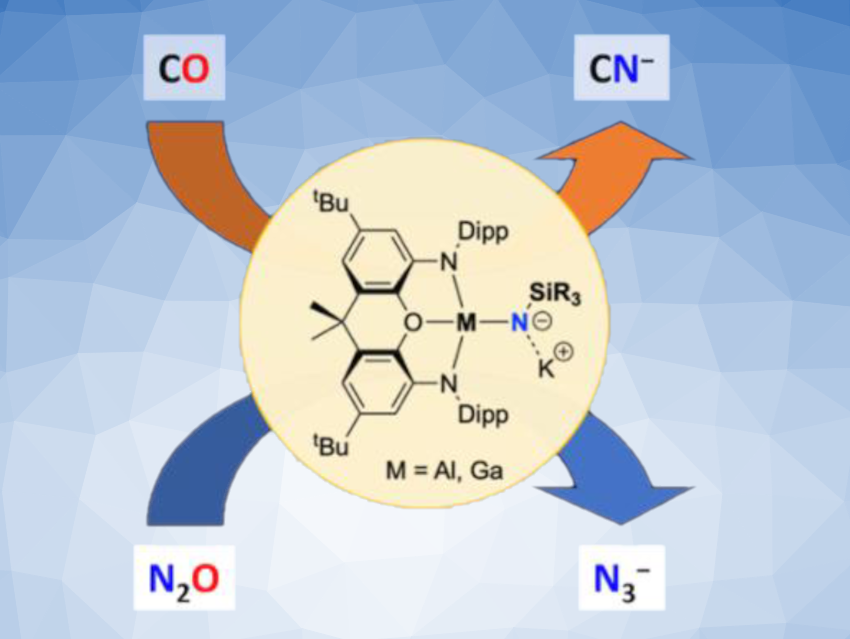
Complexes of group-13 elements such as aluminium with imide ligands (NR2–) can be interesting research targets, e.g., for investigations of potential metal–nitrogen multiple bonding or as precursors for materials that could, for example, store hydrogen or serve as semiconductors. However, terminal imide complexes such as those of the type Ln(X)Al(NR) are often very labile and difficult to isolate.
Jose M. Goicoechea, Simon Aldridge, University of Oxford, UK, and colleagues have synthesized terminal aluminium and gallium imides of the type K[(NON)M(NR)] for the first time (NON = 4,5-bis(2,6-diisopropyl-anilido)-2,7-di-tert-butyl-9,9-dimethyl-xanthene, R = silyl or boryl substituents, example pictured). The team used anionic aluminium(I) and gallium(I) reagents (“aluminyl” or “gallyl” reagents) such as K2[(NON)Al]2 and reacted them with silyl or boryl azides.
N(SiiPr3) and N(boryl)-type complexes were extremely labile, while phenylsilylimido derivatives offer greater stabilization. N(SitBuPh2)-substituted complexes turned out to be a usable compromise between stability and solubility, and the aluminium complex with this substituent was fully characterized. The synthesized compounds feature minimal π-bonding between the imide ligand and aluminium/gallium. The team found that both aluminium and gallium silylimides can act as sources of nitride ions(N3–) in reactions with CO or N2O.
- Aluminium and gallium silylimides as nitride sources,
Andreas Heilmann, Artemis M. Saddington, Jose M. Goicoechea, Simon Aldridge,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302512