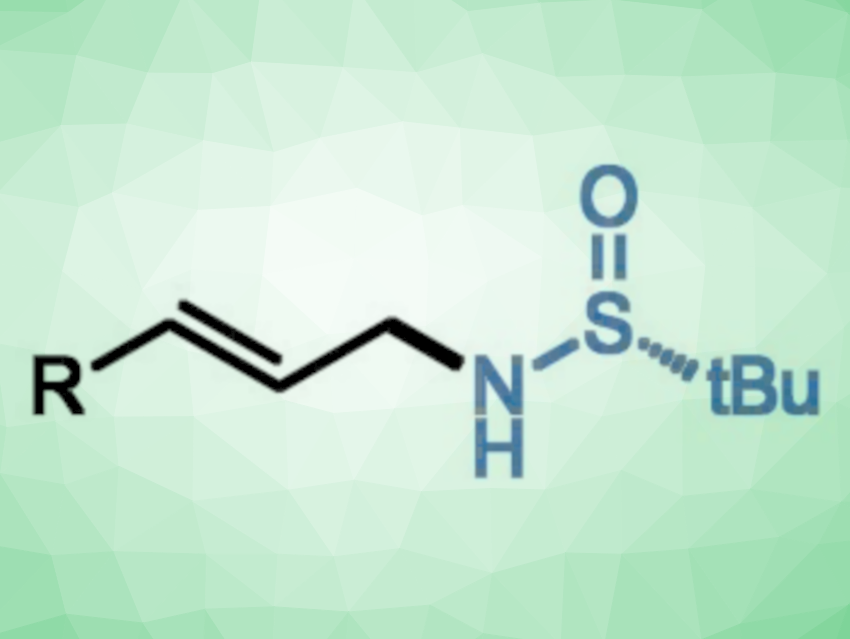
Allylic amines are useful intermediates in organic synthesis. The amination of allylic alcohols is a straightforward route for their preparation. Traditionally, the amination of allylic alcohols via the Mitsunobu reaction requires stoichiometric amounts of activating reagents. The Tsuji-Trost allylic substitution provides a catalytic alternative. However, a pre-activation of allylic alcohols is generally required, and the reaction can have selectivity issues between linear and branched products.
Wei Ma, Shaanxi Normal University, China, and Ningxia Medical University, Yinchuan, China, Chao Wang, Shaanxi Normal University, and colleagues have found that allylic alcohols can react with chiral tert-butysulfinamide to give allylic amides in a reaction catalyzed by iron complexes (pictured below). The team reacted a broad range of allylic alcohols with tert-butysulfinamide, using a PNP-type pincer complex of iron as the catalyst, NaBHEt3 as a hydride source, Cs2CO3 as a base, and cyclohexane as the solvent. The reactions were performed at 50 °C.

The desired products were obtained in moderate to excellent yields with exclusive linear selectivity and retention of enantioselectivity. The reaction proceeds via a borrowing hydrogen pathway. It does not require a pre-activation of the allylic alcohol and produces water as the only by-product. The work shows that borrowing hydrogen catalysis can offer an alternative to the Tsuji-Trost allylic substitution.
- Fe‐Catalyzed Amidation of Allylic Alcohols via Borrowing Hydrogen Catalysis,
Xiaoyun Wu, Wei Ma, Weijun Tang, Dong Xue, Jianliang Xiao, Chao Wang,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201829
Also of Interest
- What is Borrowing Hydrogen Catalysis?,
ChemistryViews 2016.
https://doi.org/10.1002/chemv.201600081
A green chemistry approach to activating alcohols