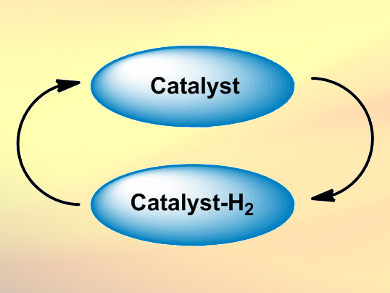
Borrowing hydrogen catalysis, also called hydrogen autotransfer or dehydrogenative activation, is a method in organic chemistry to activate, for example, alcohols. Alcohols are not very reactive, in contrast to their oxidation products, aldehydes and ketones.
These carbonyl compounds, which technically differ from the alcohols by one H2 molecule, are much better electrophiles and can be used in a variety of reactions. Borrowing hydrogen catalysis uses this “chemical detour” as method of activation.
Example Reaction
- A catalyst “borrows” H2 from an alcohol, forming a carbonyl compound.
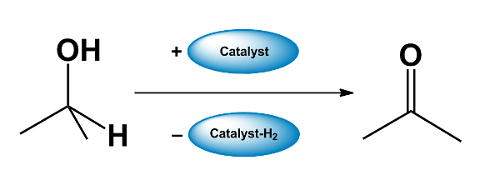
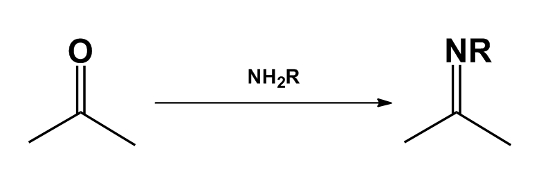
- The carbonyl compound takes part in a reaction which would not be possible for the alcohol.
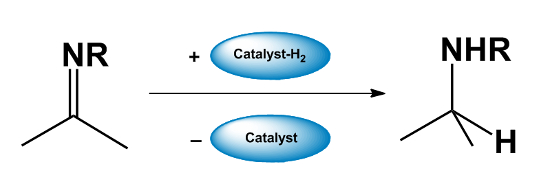
- The catalyst “returns” the hydrogen to the reaction product afterward.
The overall process allows alcohols to be converted into amines, to form C–C bonds, or to be functionalized at the β-position. The catalysts are usually transition metal complexes, e.g., Ru, Ir, or Rh compounds. In addition to alcohols, borrowing hydrogen catalysis can also be applied to amines and even alkanes.
Sources
- Applications of Acceptorless Dehydrogenation and Related Transformations in Chemical Synthesis,
C. Gunanathan, D. Milstein,
Science 2013, 341, 1229712–1229712.
DOI: 10.1126/science.1229712 - Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis,
Graham E. Dobereiner, Robert H. Crabtree,
Chem. Rev. 2010, 110, 681–703.
DOI: 10.1021/cr900202j - Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds using Alcohols and Amines as Electrophiles,
Gabriela Guillena, Diego J. Ramón, Miguel Yus,
Chem. Rev. 2010, 110, 1611–1641.
DOI: 10.1021/cr9002159 - Borrowing Hydrogen in the Activation of Alcohols,
Malai Haniti S. A. Hamid, Paul A. Slatford, Jonathan M. J. Williams,
Adv. Synth. Catal. 2007, 349, 1555–1575.
DOI: 10.1002/adsc.200600638
Also of Interest
- Borrowing Hydrogen to Make Branched Ketones,
ChemistryViews.org 2016.
α-Alkylation of methylene ketones using alcohols