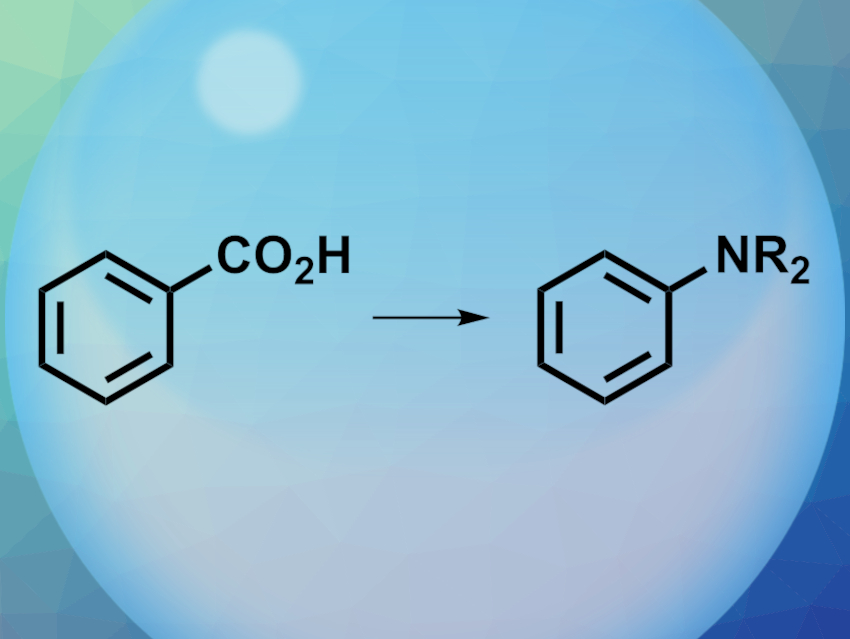
Aromatic amines are useful building blocks in organic synthesis. There are several methods to synthesize aryl amines. One approach is based on decarbonylative amination, i.e., carboxylic acids are converted to amines (pictured). However, so far, there had been no reported direct, catalyst-free transformations of benzoic acid to aniline in water.
Microdroplets of water can serve as tiny chemical reactors with properties that differ from bulk water. For example, there can be large numbers of hydroxyl radicals at the water–gas interface of microdroplets, produced by an electric field or contact electrification.
Yifan Meng, Richard N. Zare, Stanford University, CA, USA, and Elumalai Gnanamani, Indian Institute of Technology, Roorkee, India, have performed a one-step synthesis of aniline from benzoic acid in water via decarboxylation, using in-situ generated hydroxyl radicals. The team dissolved benzoic acid and ammonium hydroxide in water. The resulting solution was injected into a droplet sprayer and nebulized, while a positive voltage was applied to the sprayer. The products prepared in the microdroplets were directly detected using high-resolution mass spectrometry.
The researchers found that small amounts of aniline were obtained. The scope of the reaction also includes electron-rich, electron-deficient, and polyaromatic carboxylic acids as well as primary and secondary amines. The transformation proceeds at room temperature and does not require organic solvents or metal catalysts.
The team proposes a reaction mechanism that involves the formation of NR2OH via a reaction of the amine with hydroxyl radicals. This intermediate reacts with the carboxylic acid to form an isocyanate. The desired aryl amine is then obtained via hydrolysis and CO2 loss.
- Catalyst-Free Decarboxylative Amination of Carboxylic Acids in Water Microdroplets,
Yifan Meng, Elumalai Gnanamani, Richard N. Zare,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c12236