Crown ethers are mostly neutral macrocyclic compounds with remarkable molecular recognition properties. They are composed of a cyclic backbone of repeating units with donor atoms positioned at regular intervals along the ring. These donor atoms can coordinate metal ions and determine the reactivity of the crown ethers.
Marian Hebenbrock. University of Münster, Germany, and colleagues have developed anionic boraza-crown ethers (pictured), featuring two different types of donor atoms (N and O) and an intrinsic charge compensation for metal ions in an anionic boronate linking unit. These boraza-crown ethers are easily accessible via a reaction of dioxazaborocanes with different alkoxides. The dioxazaborocane was synthesized in a yield of 97 % from phenylboronic acid via a condensation reaction with N-methyldiethanolamine. Then it was reacted with sodium benzyloxide, sodium ethoxide, sodium tert-butoxide, or potassium benzyloxide to obtain the desired dimeric products.
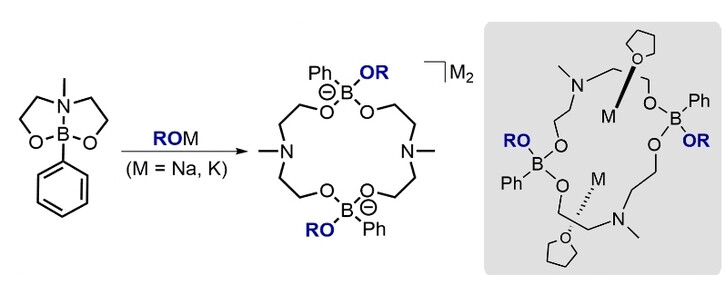
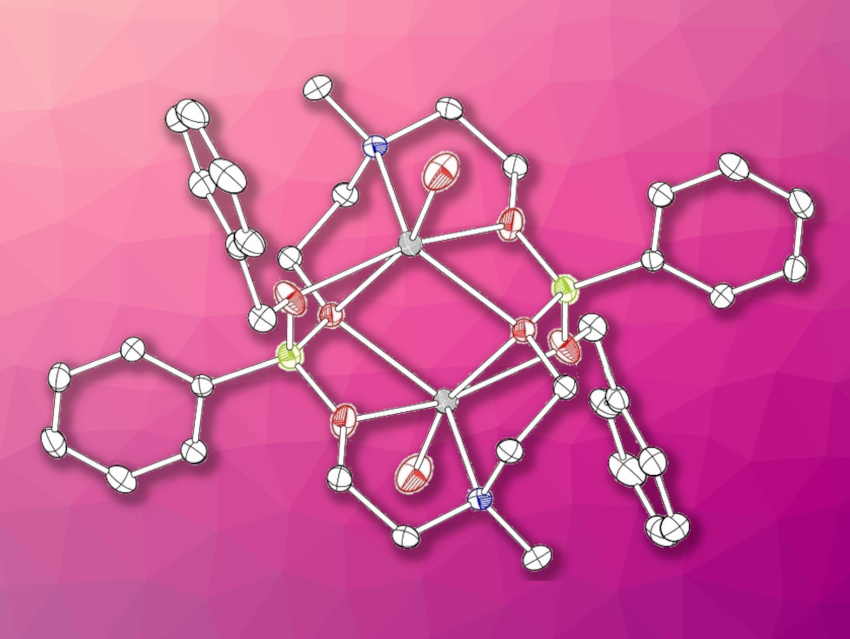
The dimeric state of the boraza-crown ethers in the solid state was confirmed by X-ray diffraction, and by DOSY NMR spectroscopy in solution. This structure makes them good candidates for metal-ion coordination in solution. Unlike other crown ethers, the boraza-crown ethers can coordinate two singly charged metal ions simultaneously. The team also investigated the reactivity of the complexes and found that they can engage in cross-coupling reactions.
- Anionic Boraza‐Crown Ethers,
Tabea Lenz, Alexander Hepp, Marcus Layh, Marian Hebenbrock,
Eur. J. Inorg. Chem. 2023.
https://doi.org/10.1002/ejic.202300401