Analogues of benzene with heavier main-group elements in the place of one or more carbon atoms can be interesting research targets. However, such heteroarenes with comparatively heavy elements such as antimony, for example, can be challenging to synthesize due to their high reactivity and the weak C=Sb π-interaction. While phosphabenzene derivatives are used, e.g., as ligands, heavier stibabenzene and bismabenzene derivatives are difficult to stabilize.
Rajendra Ghadwal, University of Bielefeld, Germany, and colleagues have synthesized the first 1,4-distibinine-1,4-diide compound, [(ADC)Sb]2 (5, pictured below), based on an anionic dicarbene (ADC = PhC{N(Dipp)C}2, Dipp = 2,6-iPr2C6H3). The compound can be regarded as a base-stabilized cyclic bis-stibinidene, in which each of the Sb atoms bears two lone pairs of electrons.
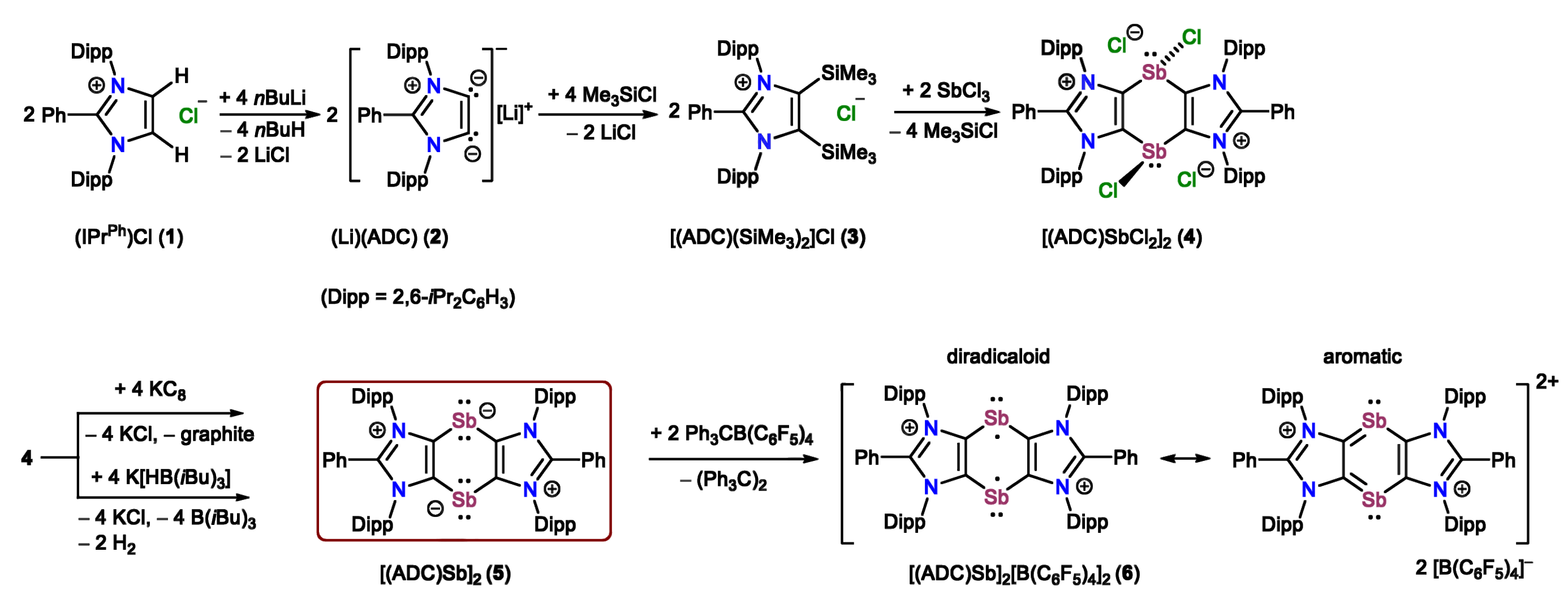
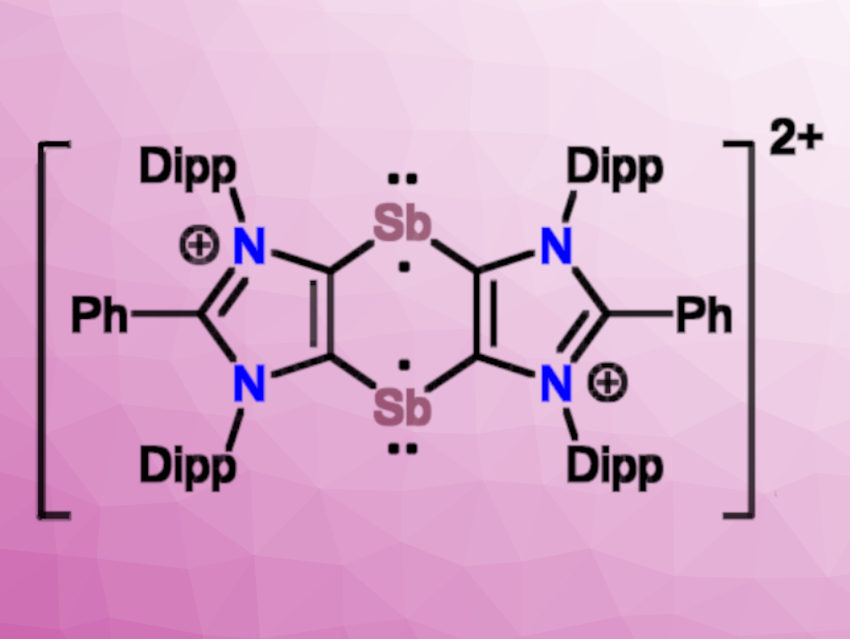
Compound 5 undergoes a two-electron oxidation with Ph3C[B(C6F5)4] to give [(ADC)Sb]2[B(C6F5)4]2 (6, pictured above), which is a 1,3-imidazole-fused 1,4-distibinine derivative with a central C4Sb2 ring that formally contains 6 π-electrons. This product has a diradicaloid character, partly because the local aromatic stabilization in the imidazole rings reduces the local aromaticity in the C4Sb2 ring (diradicaloid and aromatic resonance structures pictured above). According to the researchers, compound 6 is the first example of a stable 1,4-distibabenzene derivative so far.
- Isolation of an Annulated 1,4‐Distibabenzene Diradicaloid,
Henric Steffenfauseweh, Dennis Rottschäfer, Yury V. Vishnevskiy, Beate Neumann, Hans-Georg Stammler, Dariusz W. Szczepanik, Rajendra Ghadwal,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202216003