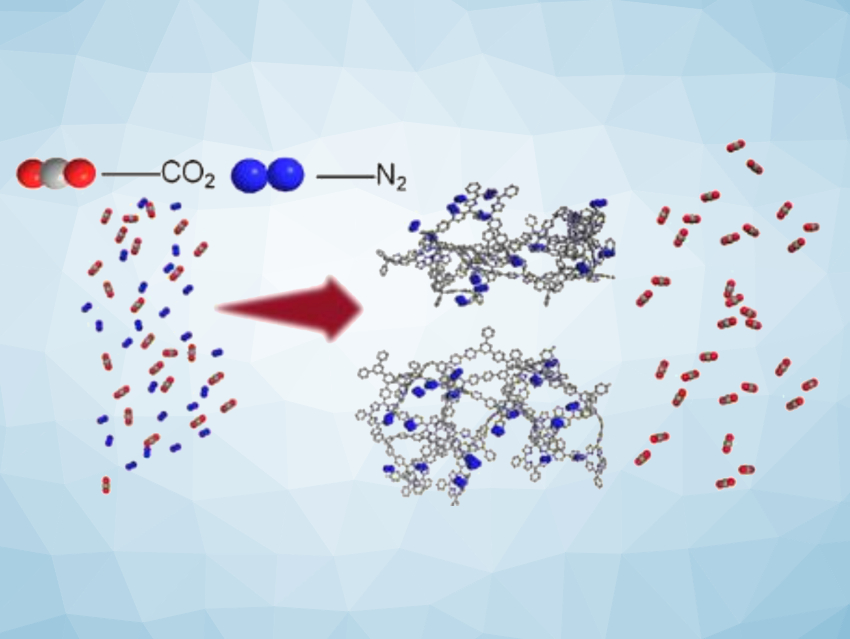
The increasing concentration of CO2 in the atmosphere poses a serious environmental threat. The capture of CO2 from industrial process emissions is, thus, important for the mitigation and prevention of the disruptive effects of global warming. Porous aromatic frameworks (PAFs) with a high specific surface area and a rich pore structure have proven to be highly efficient in adsorbing and separating various gas molecules.
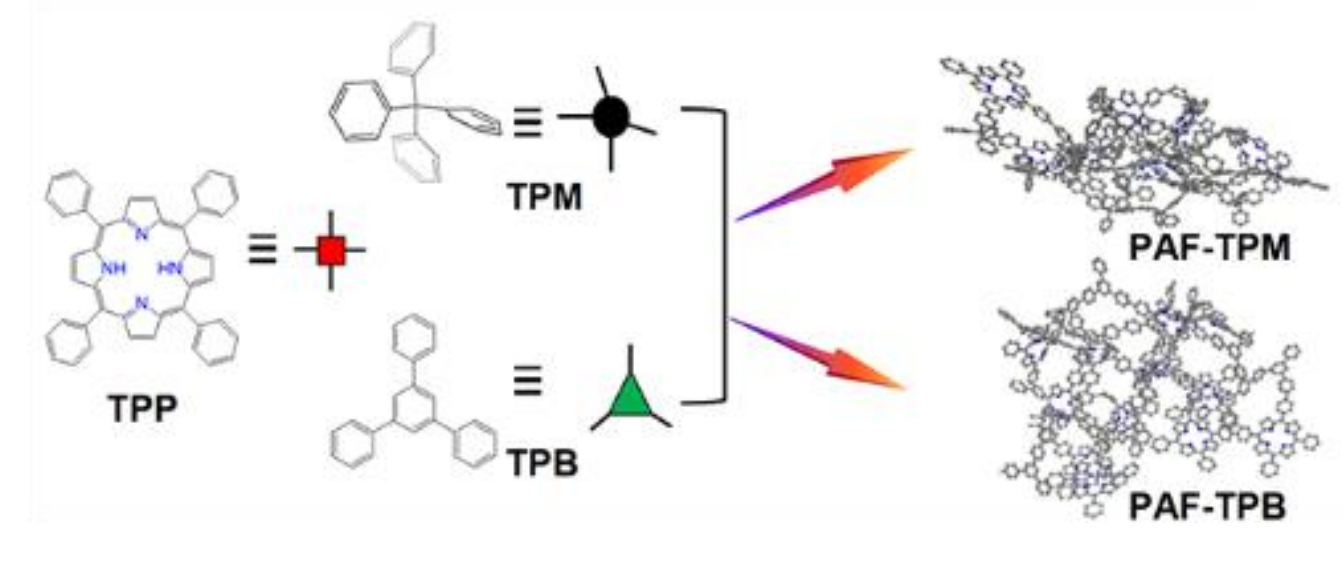
Yunhui Yang, Shuang Meng, Yunnan Normal University, Kunming, China, and colleagues have synthesized the porphyrin-based porous aromatic frameworks PAF-TPB (TPB = 1,3,5-triphenylbenzene) and PAF-TPM (TPM = tetraphenylmethane) via a Scholl reaction. The materials can be used for CO2 adsorption separation. They were synthesized from 5,10,15,20-tetraphenylporphyrin (TPP) and either TPB or TPM using AlCl3 in CHCl3 at 60 °C (pictured below).
According to the researchers, the high specific surface area of the materials is advantageous for efficient gas adsorption. PAF-TPM shows better CO2/N2 separation at low pressure (150 mbar), while PAF-TPB shows better performance at higher pressure (1 bar). In particular, PAF-TPB achieves a CO2/N2 separation efficiency of up to 100.9 at 1 bar and 273 K. Overall, the work can provide useful insight for the development of porous organic materials for the adsorption separation of CO2/N2.
- Porphyrinic Porous Aromatic Frameworks for Carbon Dioxide Adsorption and Separation,
Jierui Yang, Huiting Qiu, Long Huang, Shuang Meng, Yunhui Yang,
ChemPlusChem 2023.
https://doi.org/10.1002/cplu.202300292