Non-proteinogenic α,α-disubstituted α-amino acids could have applications, e.g., in biochemical research and drug discovery. The α-allylation of α-amino acids is one convenient approach to constructing such compounds. Often, imines are used as substrates in the α-functionalization of amine compounds, which requires additional steps for amine protection and deprotection. Using aldehydes as part of the catalytic system can allow the α-functionalization of amines without additional protection/deprotection steps. However, enantioselective variants of this type of reaction generally require chiral aldehydes. Catalytic systems that can use commercially available achiral aldehydes for asymmetric α-allylations would be useful.
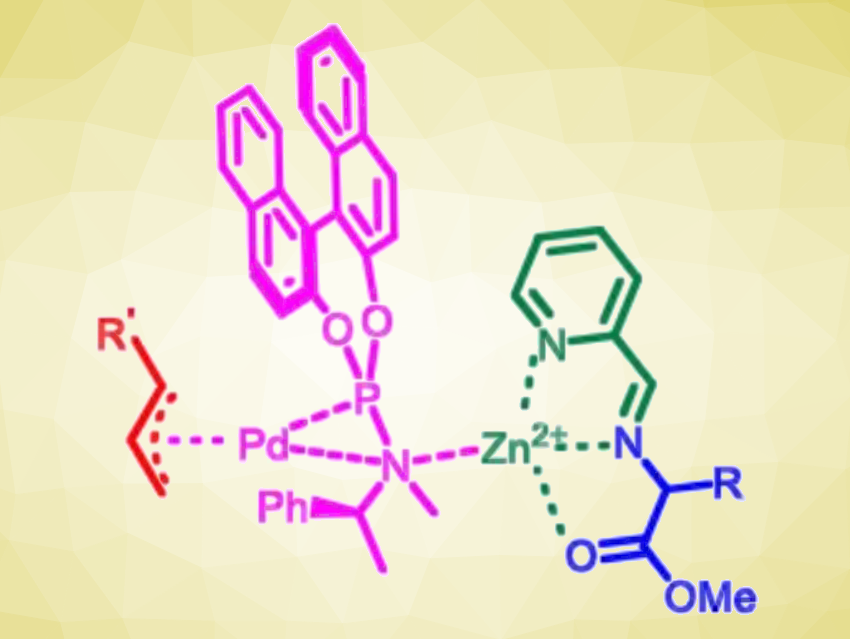
Yan Liu, Can Li, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and colleagues have developed a synergistic, three-part catalyst system based on achiral picolinaldehyde, Zn(II), and a chiral palladium complex for the enantioselective α-allylation of N-unprotected amino esters (pictured below). The team used allylic carbonates or vinyl benzoxazinanones as substrates and obtained the desired α-allyl α-amino esters in high yields (up to 96 %) and with high enantioselectivities (up to 98 % ee).
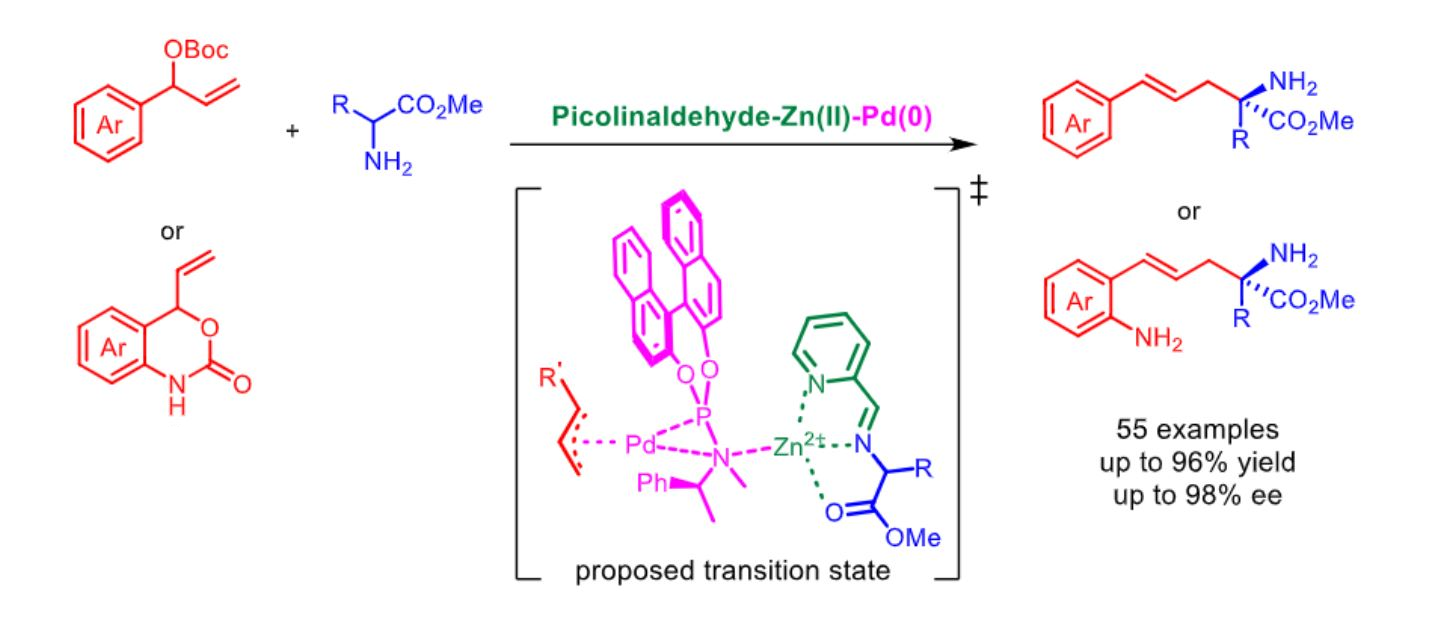
The researchers propose that the coordination of Zn(II) with the Schiff base that is formed as an intermediate (pictured in green/blue) enhances the acidity of the α-C–H bonds in the amino esters. This favors the desired α-allylation over N-allylation. The method showed good functional group tolerance, and the reactions proceed under mild conditions. Overall, the developed catalytic strategy could serve as a tool for accessing enantioenriched α,α-disubstituted α-amino acids.
- Picolinaldehyde‐Zinc(II)‐Palladium(0) Catalytic System for the Asymmetric α‐Allylation of N‐unprotected Amino Esters,
Qian Li, Yan Liu, Can Li,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301348