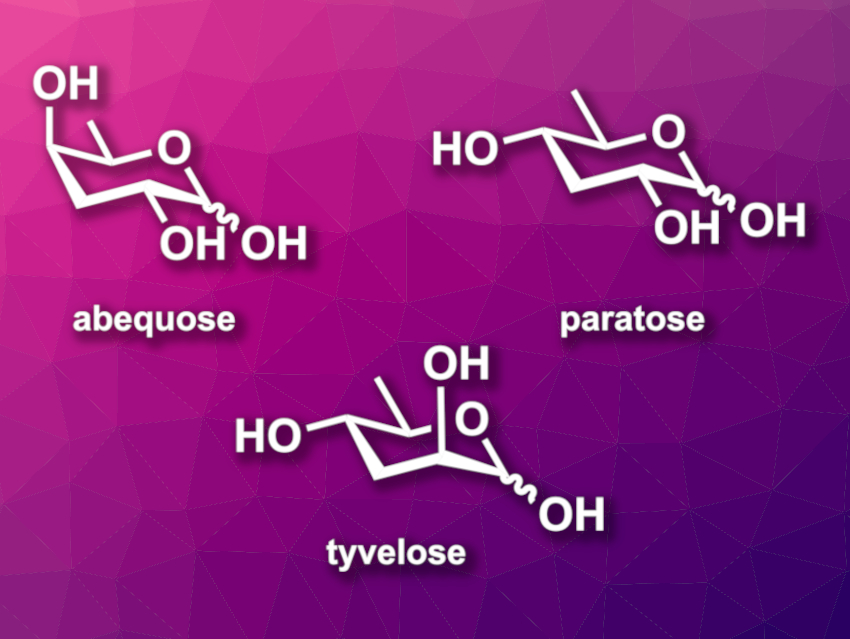
Deoxygenated carbohydrates can be found, e.g., in a range of bioactive natural products. 3,6-Dideoxy sugars, such as abequose, paratose, or tyvelose (pictured), for example, decorate the lipopolysaccharides of some bacteria. They are also found in signaling molecules. 3,6-Dideoxy sugars are not generally commercially available, so methods for their synthesis are useful. Many routes start from commercially available carbohydrates.
Todd L. Lowary, The University of Alberta, Edmonton, Canada, Academia Sinica and National Taiwan University, both Taipei, Taiwan, and colleagues have developed an enantioselective approach to the synthesis of 3,6-dideoxy sugars and used it to prepare abequose, paratose, and tyvelose. The team started from 2-acetylfuran, which underwent an enantioselective reduction of the ketone group to give an alcohol. This was followed by an Achmatowicz rearrangement to obtain a pyranone intermediate.
This intermediate was transformed to either an α- or β-glycoside via a diastereoselective acylation using isobutyric anhydride in the presence of (+)-benzotetramisole or (−)-benzotetramisole, respectively. The resulting glycosyl esters were used to glycosylate 4-methoxybenzyl (PMB) alcohol to obtain protected α- or β-glycosides.
The C2 substituent was then introduced stereoselectively using a Michael addition, giving 3,6-dideoxy-4-keto sugar derivatives. The ketone groups can be reduced to give the desired 3,6-dideoxy sugars. According to the researchers, the approach is the first enantioselective synthesis of these monosaccharides from an achiral starting material.
- A De Novo Route to 3,6-Dideoxy Sugars,
Sheng Yang, Chun-Jui Chu, Todd L. Lowary,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02349