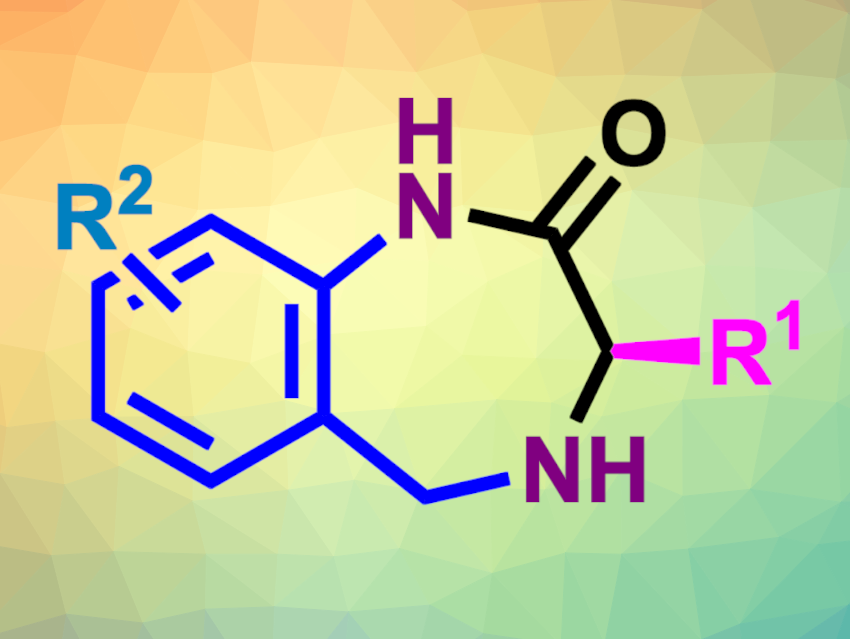
Tetrahydro-1,4-benzodiazepin-2-ones, which contain a seven-membered heterocycle with two nitrogen atoms fused to a benzene ring, are structures found, e.g., in pharmaceutically active compounds. Benzodiazepines are often used in central nervous system treatment, for example, against anxiety. This class of compounds is generally synthesized via stereoselective stepwise approaches, relying on natural L-α-aminoacids as starting materials. New streamlined catalytic asymmetric strategies with a wide substrate scope would be useful.
Alessandra Lattanzi, University of Salerno, Italy, and colleagues have developed a one-pot enantioselective route to tetrahydro-1,4-benzodiazepin-2-ones (general product structure pictured above) using commercially available reagents, a quinine-derived urea catalyst, and a single solvent. The products were prepared from readily available aldehydes, phenylsulfonylacetonitrile, and 2-(aminomethyl)anilines, with epi-quinine derived urea (eQNU) as the organocatalyst. The team used an organocatalyzed Knoevenagel reaction/asymmetric epoxidation/domino ring-opening cyclization (DROC) sequence, involving a key epoxide intermediate (pictured below).
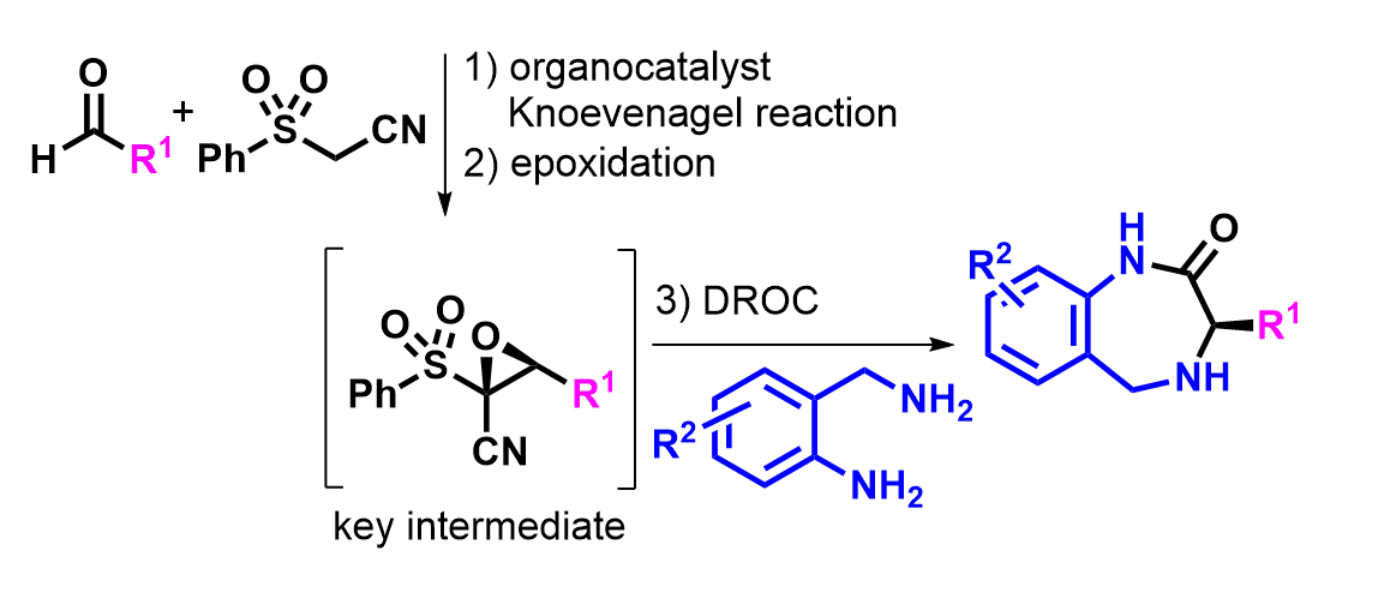
The desired heterocycles were obtained under mild conditions in satisfactory overall yields and good to high enantioselectivity (up to 98 % ee). The work could have applications in the asymmetric synthesis of seven-membered heterocycles that are useful in medicinal chemistry and can undergo further transformations.
- Catalytic Asymmetric Approach to 1,3,4,5‐Tetrahydro‐1,4‐benzodiazepin‐2‐ones in One‐Pot,
Vincenzo Battaglia, Sara Meninno, Armando Astone, Alessandra Lattanzi,
ChemCatChem 2024.
https://doi.org/10.1002/cctc.202400481