Reaction with allyl aryl ethers using Co(III) catalysis
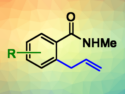

Reaction with allyl aryl ethers using Co(III) catalysis
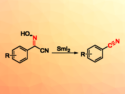
Reaction involves cleavage of the N–O bond in cyanoximes promoted by SmI2
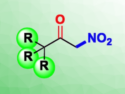
Environmentally benign synthesis requires only a base and molecular oxygen
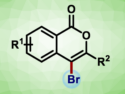
Brominative annulation of 2‐alkynylaryloate esters using an in-situ-generated bromoiodane
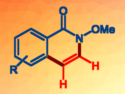
Rhodium(III)-catalyzed C–H activation and annulation in ethanol
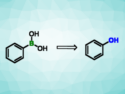
Reaction under UV-A light gives phenols and aliphatic alcohols
Tetrabutylammonium acetate acts as base, ligand, and solvent, providing a simple and efficient synthesis route
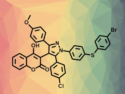
Condensation of cyclic β-diketones, arylglyoxals, and arylhydrazones
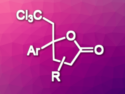
Regio- and stereoselective reaction gives a variety of lactones with a tetrasubstituted carbon and a trichloromethyl group
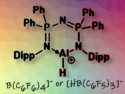
A wide range of imines were hydrosilylated using a variety of silanes, catalyzed by electronically unsaturated cationic aluminum hydrides