Pyrazole derivatives substituted with, e.g., coumarin units, β-diketones, or aryl/hetero-aryl groups can show a wide range of bioactivities and useful photophysical properties. Therefore, the development of efficient methods for the synthesis of this type of pyrazole derivatives is an interesting research target. Existing syntheses can have drawbacks such as the need for transition-metal catalysts, limited substrate scopes, or harsh reaction conditions.
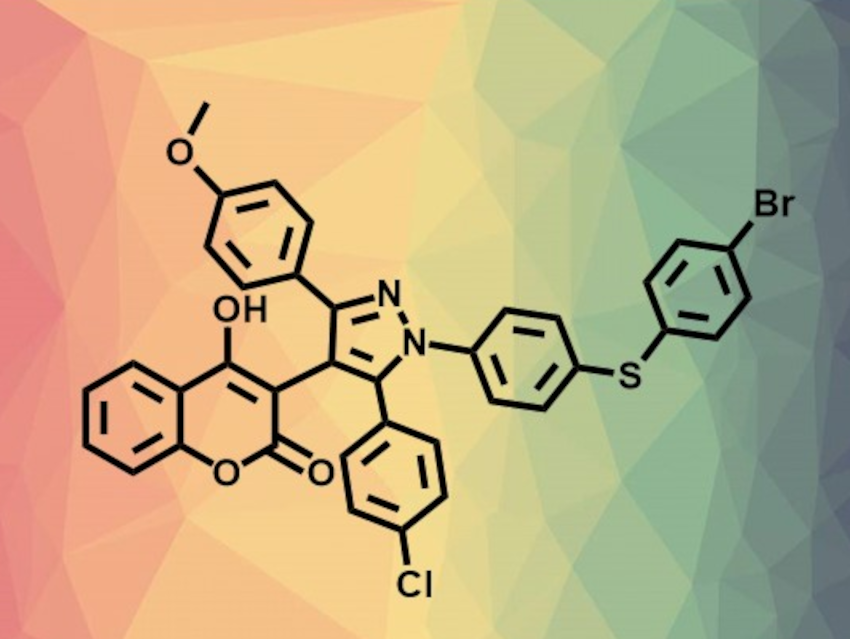
Animesh Pramanik, University of Calcutta, India, and colleagues have developed a method for the one-pot, three-component, atom-economic synthesis of multifunctionalized pyrazole derivatives (example product pictured above). The team used a simple p-toluenesulfonic acid (p-TsOH)-catalyzed of readily available cyclic β-diketones (such as dimidone, 4-hydroxycoumarin, or 2-hydroxy-1,4-naphthoquinone), arylglyoxals, and arylhydrazones (pictured below). The reactions were performed in dimethylformamide (DMF) at 70 °C and gave various aryl- and cyclic β-diketone-substituted pyrazole derivatives.
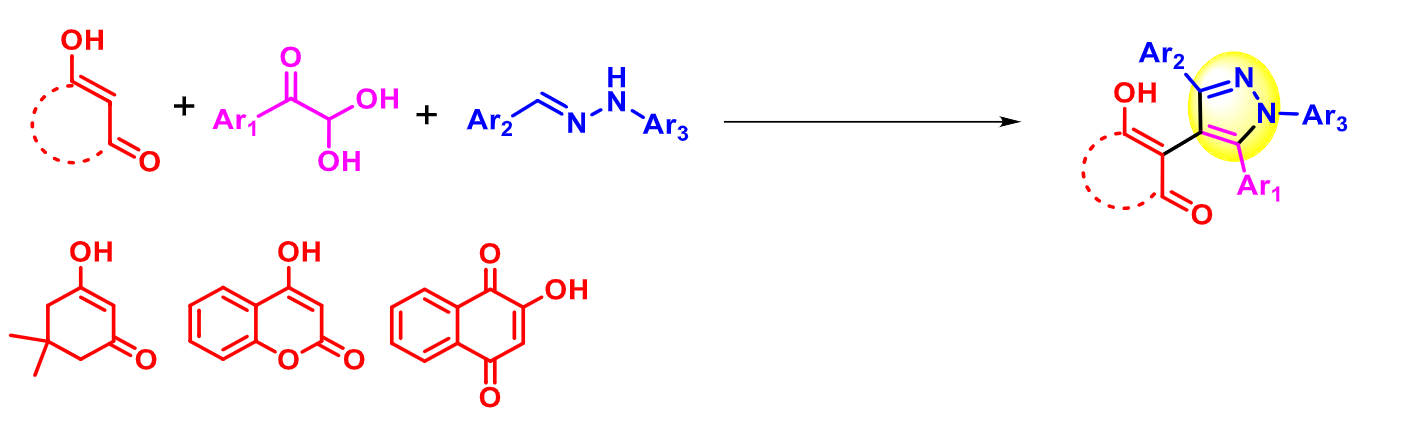
The method features a broad substrate scope, good functional group tolerance, and high yields (even in a gram-scale reaction). The synthesis provides an easy way to incorporate, e.g., biologically important N-diarylsulfide/selenide units, various enolizable cyclic β-diketones, and other heterocycles in addition to pyrazole. This could be helpful for the development of new pyrazole derivatives, e.g., for the pharmaceutical industry.
- Efficient Synthesis of Fully Substituted and Diversely Functionalized Pyrazoles through p‐TSA Catalyzed One‐Pot Condensation of Cyclic β‐Diketones, Arylglyoxals and Arylhydrazones,
Arun Dhurey, Subhro Mandal, Animesh Pramanik,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300770