A mild, efficient system for selectively deprotecting and modifying N-heterocycles in a single step


A mild, efficient system for selectively deprotecting and modifying N-heterocycles in a single step

BioMed X Institute opens its global crowdsourcing platform to help principal investigators in biomedical research who have lost their NIH funding to find alternative project sponsors among the global pharmaceutical industry
The survey is conducted by EuChemS and the European Young Chemists’ Network (EYCN), supported by Chemistry Europe, the Royal Society of Chemistry (RSC), and the American Chemical Society (ACS)

The Swiss Chemical Society (SCS) awards the F. Hoffmann-La Roche team involved in the commercial manufacturing process of Divarasib, an KRAS G12C inhibitor for cancer treatment

Merck and Zebra collaborate to address challenges around product verification, authenticity, and trust

Exploring cage bicyclic hydrocarbons as bioisosteres for novel fragrances

Can you guess the famous scientist from the description?

Join us as we explore an astonishing lost element, the need for more self-assured chemists, and the unforgettable story of Fridolin E. Coli
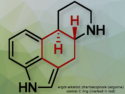
A previously unobserved enzymatic strategy that expands the understanding of metalloenzyme catalysis and may guide the design of new biocatalysts for pharmaceutical synthesis

Stabilization of the cyclo-P₅⁻ anion in the solid state through complexation of the alkaline metal cation with [2.2.2]cryptand, providing the first crystallographic characterization and detailed spectroscopic analysis