A lack of understanding of the formation of urea oxidation products hinders the improvement of catalysts for urea oxidation in water, which is essential for the sustainable management of urea pollution. This is critical for applications such as artificial kidney dialysis, non-enzymatic electrochemical sensors, and water reclamation for long-duration space missions.
Stephen Tatarchuk and Leanne D Chen, University of Guelph, Canada, and colleagues have analyzed the urea oxidation pathways on a (0001) β-Ni(OH)2 surface using density functional theory to gain a more complete atomic understanding of the urea oxidation reaction (UOR) at the surface of NiOx electrocatalysts.
Mr. Tatarchuk, what have you done?
Our research highlights the thermodynamic barriers required to form each product of the urea oxidation reaction on a simplified model surface in the presence and absence of a Cu surface dopant to help improve the overall mechanistic understanding of this reaction. We conducted quantum chemical simulations with density functional theory to calculate the energy barriers required to form nitrogen gas, nitrite, and nitrate during urea oxidation reaction on a model of a nickel hydroxide electrode surface.
Through this framework, we evaluated the possible elementary steps that can occur at the surface of a urea oxidation reaction catalyst, considering binding conformations through either the oxygen or nitrogen atoms in urea. The results of this enabled us to formulate free energy diagrams for the lowest energy steps required to form each product.
Why are you doing this?
In light of the recent observations of nitrite and nitrate formation during the urea oxidation reaction in addition to nitrogen gas, we were interested in understanding which elementary reaction steps would provide the lowest energy pathway for each product. Once there is a strong understanding of which steps control how much energy is required to produce each product, it opens the doors to improve our ability to predict what type of catalyst modulation strategies to pursue and help deduce the origin of increased selectivity with previously explored approaches at a more fundamental level.
What is new and cool about it?
We examine the formation of different urea oxidation reaction products, considering both N-bound and O-bound reaction intermediates on a nickel hydroxide model surface in the absence and presence of a Cu dopant. These findings help provide some much-needed insight into the selectivity between the reaction pathways of the urea oxidation reaction, and help refine which approaches should receive more focus during catalyst design.
Key observations between our simulations and experimental observations show qualitative agreement in many aspects, indicating that our simplified surface model is appropriate for exploring different approaches to tuning pathway selectivity from first principles.
What are your key findings?
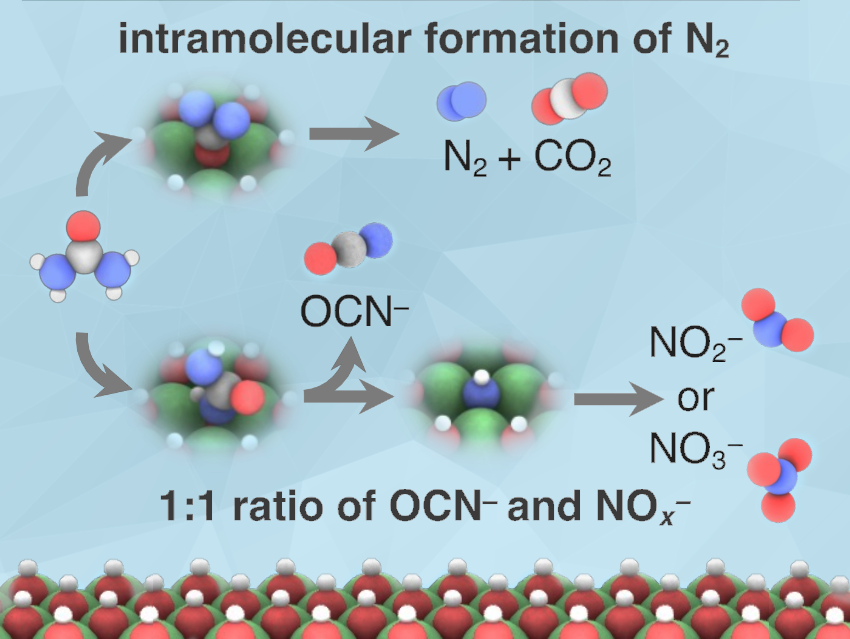
Our research shows the thermodynamic barriers required for the formation of different urea oxidation reaction products in the presence and absence of a Cu surface dopant. For the formation of N2, we found that the previous experimental observation that the main pathway for the formation of N2 occurs through an intramolecular N—N coupling step rather than an intermolecular N—N coupling step appears to be driven by the fact that the intramolecular pathway has a lower thermodynamic barrier due to the surface intermediates involved.
Beyond modeling N2 formation, we explored the formation of NO2– and NO3– where our calculations support the previous observation that NO2–/NO3– form in a 1:1 ratio with OCN–. Interestingly, in this work, we observed that the selectivity appears to be influenced by the atom through which the reaction intermediates bind to the surface (N or O) and is sensitive to which deprotonation steps are energetically favored on the catalyst surface.
Extending this analysis to the Cu-doped system, we found that the experimental increase in N2 selectivity in the presence of a Cu dopant may be driven by increasing the thermodynamic barriers to form NO2– rather than lowering the barriers to form N2.
What specific applications do you envision? What is the longer-term vision for your research?
While many aspects of the urea oxidation reaction are yet to be fully understood, this reaction shows promise in applications toward water purification, agricultural emission reduction, enzyme-free sensors, sustainable fertilizer production, paired-electrolysis, and as a hydrogen carrier. Additionally, the versatility of the urea oxidation reaction may lead it to play a critical role in addressing some of the challenges of long-term space missions related to water reclamation, energy production, and fertilizer production.
The longer-term vision of our research will focus on establishing a systematic framework that can model the thermodynamic and kinetic barriers of the urea oxidation reaction, not only including higher oxidation state surfaces but also capturing the influence that solvation and applied electric fields have on the stability of various reaction intermediates.
Thank you very much.
The paper they talked about:
- Understanding the Mechanism of Urea Oxidation from First-Principles Calculations,
Stephen W. Tatarchuk, Rachelle M. Choueiri, Alexander J. MacKay, Shayne J. Johnston, William M. Cooper, Kayla S. Snyder, Jury J. Medvedev, Anna Klinkova, Leanne D. Chen,
ChemPhysChem 2024.
https://doi.org/10.1002/cphc.202300889

Stephen Tatarchuk is a Ph.D. student in the group of Assistant Professor Leanne D. Chen, Department of Chemistry, University of Guelph, ON, Canada, and the lead author of the paper.