Molecular recognition is fundamental to both supramolecular chemistry and biological processes. Integrating tetraphenylethylene (TPE) into cyclic skeletons presents a promising strategy for tailoring inherently luminescent macrocycles, bridging supramolecular macrocyclic chemistry with aggregation-induced emission materials. However, embedding this propeller-shaped fluorophore usually induces “twisted and flattened” cavity conformations, resulting in the loss of molecular recognition function in dilute solutions, especially in TPE-embedded pillar[6]arenes. Therefore, facile structural modification of such macrocycles for effective dual-state recognition in both dilute solutions and aggregate states remains an unresolved challenge.
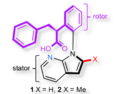
Inspired by natural enzymes, Jialin Xie, Chunman Jia, Hainan University, China, and colleagues have developed three TPE-based cyclo[6]arenes (termed TPz, TDz, and TTz) with endo-functionalized cavities containing inward-directed diazine motifs (pyrazine, pyridazine, and phthalazine) that act as H-bond acceptor sites.
- Electrostatic potential maps revealed that subtle structural variations among diazine motifs substantially modulate intracavity charge distribution with increasing electron density from TPz to TTz.
- SCXRD (Single Crystal X-ray Diffraction) analysis verified that the diazine motifs adopt equatorial conformation with N atoms oriented toward cavities as endo-binding sites.
- Systematic host–guest binding studies revealed that internal microenvironment variations translate into disparate host–guest affinities, with TTz showing the optimal performance.
- Unlike TPz’s single aggregate-state recognition capability, 1,2-diazine-modified TDz and TTz show dual-state functionality, enabling both size-selective cation binding in dilute solutions and sensitive fluorescence detection of nitrophenol pollutants in aggregate states via a static-quenching mechanism driven by photo-induced electron transfer.
Overall, this study provides new insights into the underutilized 1,2-diazine motifs as effective H-bond acceptors and offers a versatile platform for customizing biomimetic host systems with emergent functionalities.
- Diazine Endo-Functionalized Tetraphenylethylene-Based Cyclo[6]arenes for Molecular Recognition in Both Solution and Aggregate States
Nan Pan, Shuhong Liu, Weichen Tan, Linbin Yao, Jialin Xie*, Kelong Zhu, Chunman Jia*
Aggregate 2025
https://doi.org/10.1002/agt2.70171
![Diazine-Tetraphenylethylene Cyclo[6]arenes for Molecular Recognition in Solution and Aggregate States](https://www.chemistryviews.org/wp-content/uploads/2025/11/ChemistryViews-2.70171.png)