Idiopathic pulmonary fibrosis (IPF) is an irreversible and fatal lung disease characterized by continuous alveolar epithelial cell damage and extracellular matrix deposition. Current FDA-approved drugs, such as nintedanib and pirfenidone, can only slightly slow disease progression, fail to significantly extend median survival, and are associated with severe side effects. Therefore, there is an urgent need to develop novel therapeutic strategies.
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) exhibit great potential in the treatment of pulmonary fibrosis due to their anti-inflammatory and tissue repair properties. However, their application still faces multiple challenges, including low yield, high heterogeneity, and inefficient pulmonary delivery.
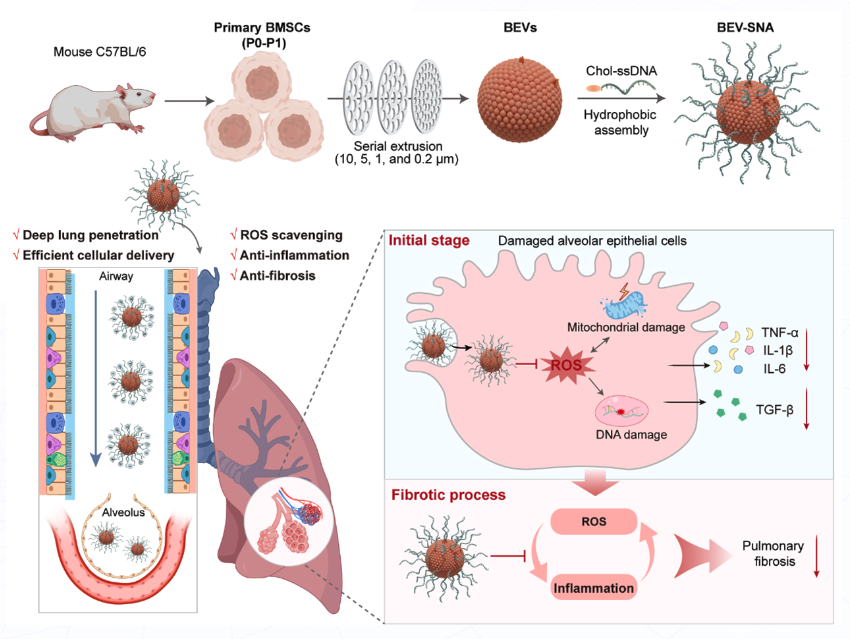
Kaizhe Wang (Ningbo Institute of Materials Technology and Engineering of Chinese Academy of Sciences, China), Zhaoxing Dong (Ningbo No. 2 Hospital, China), Jiang Li (Shanghai University, China), and colleagues developed a biomimetic extracellular vesicle spherical nucleic acid (BEV-SNA) platform for the treatment of IPF. By hydrophobically co-assembling biomimetic extracellular vesicles (BEVs) obtained through mechanical extrusion of primary mesenchymal stem cells (MSCs) with cholesterol-modified single-stranded DNA (chol-ssDNA), BEV-SNA with a three-dimensional extended structure was obtained. In MSCs of the P0-P1 generations that maintained stem cell characteristics, the yield of BEVs increased by 17.2 times compared to natural EVs. Compared to bare BEVs, BEV-SNA exhibited stronger reactive oxygen species (ROS) scavenging ability, which could reduce oxidative stress damage in the lungs. Thanks to its three-dimensional dense and negatively charged DNA shell, BEV-SNA could reduce airway mucus adsorption, achieve deep delivery into lung tissue, and be efficiently taken up by alveolar epithelial cells.
In the IPF model, BEV-SNA demonstrated multi-stage therapeutic effects, including protecting alveolar epithelial cells from ROS damage, inhibiting inflammation, and exerting anti-fibrotic effects in the late stage, effectively halting fibrosis progression and achieving a 50% survival rate. This study proposes a novel therapeutic platform combining the natural biomimetic properties of BEVs with the functional adaptability of SNAs, providing an innovative strategy for pulmonary drug delivery and the treatment of respiratory diseases.
- Lung-Penetrating Biomimetic Extracellular Vesicle Spherical Nucleic Acids for Pulmonary Fibrosis Therapy Through ROS Scavenging and Anti-Inflammatory Effects,
Saiyun Lou, Jiangpo Ma, Pan Fu, Lin Li, Jingyun Huang, Fangxue Jing, Yuhui Wang, Sihua Qian, Jianping Zheng, Jiang Li, Zhaoxing Dong, Kaizhe Wang,
Aggregate 2025.
https://doi.org/10.1002/agt2.70086