Christian Hering-Junghans, Leibniz Institut für Katalyse e.V. (LIKAT), Rostock, and Robert Kretschmer, Chemnitz University of Technology, Germany, discuss a recent study published by them and their colleagues in the European Journal of Inorganic Chemistry (EurJIC) on the ambident reactivity of the bis(gallylene), as demonstrated by its reactions with azobenzene and the triphosphirane (PMes)₃.
What did you do?
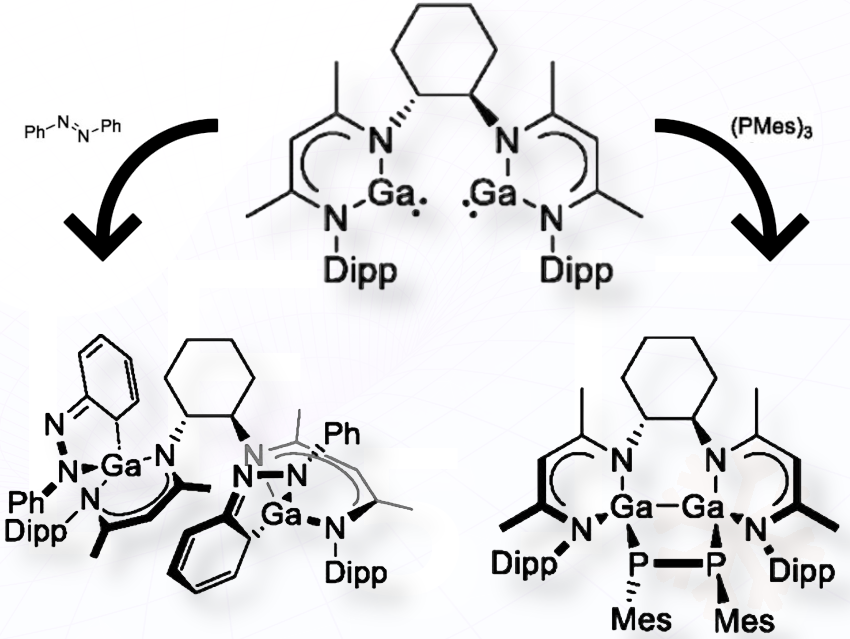
Gallylenes are highly reactive group 13 compounds with both filled and empty orbitals at the gallium center. Our team managed to combine two of such centers within one molecule. These bis(gallylenes) offer the potential for cooperativity between the two gallium centers.
In this study, we tested this potential by combining a bis(gallylene) with azobenzene and a triphosphirane (Mes₃P₃), a cyclic phosphorus compound that can deliver the diphosphene MesP=PMes, a heavier homolog of azobenzene.
Why are you interested in this?
The cooperativity between two gallium centers should allow the activation of strong bonds, such as H–H, C–H, or C–F, but also of N=N or P–P bonds in a way different from their monometallic relatives. This study aims to identify new reactivity patterns for bis(gallylenes), which could eventually result in new methodologies for the functionalization of the aforementioned bonds.
What is new and cool about this?
The simple combination of the bis(gallylene) with azobenzene results in the activation of two azobenzenes, which indicates that both Ga centers act independently. The five-membered GaN₂C₂ heterocycle is reminiscent of an intermediate usually found in nucleophilic aromatic substitution reactions, the so-called Meisenheimer complex. When the polarity of the solvent is changed from benzene to acetonitrile, this five-membered ring rearranges by shifting a hydrogen from carbon to nitrogen.
When combined with the triphosphirane Mes₃P₃, however, both Ga centers act in accord to give a heterocycle with a Ga₂P₂ core. Depending on the substrate, the reactivity can be easily switched.
What is the main significance of your results?
This work clearly shows the potential of bis(gallylenes) to afford new heterocycles, but more importantly to activate strong bonds. Depending on the substrates, different reactivity channels can be accessed.
What is the longer-term vision for your research?
Having two low-valent Ga centers that can act independently of each other, these compounds are envisioned for applications in catalysis. Due to the close proximity of the Ga atoms, the simultaneous activation of two different substrates could enable challenging bond forming processes.
What part of your work was the most challenging?
Having a cyclohexylene bridging unit between both entities results in rather complex NMR spectra; thus, product identification was the most challenging aspect.
Anything else you would like to add?
This work is a good example of a successful collaboration, involving a student who worked on it during his bachelor’s thesis in one laboratory and later completed his master’s thesis in the group of the other collaborator.
Thank you very much for sharing these insights.
The paper they talked about:
- Gallium/Group 15 Heterocycles Derived from a Bis(gallylene),
Aylin Nagel, Max Neubauer, Phil Köhler, Tobias Rüffer, Christian Hering-Junghans, Robert Kretschmer,
Eur. J. Inorg. Chem. 2025.
https://doi.org/10.1002/ejic.202500538
Christian Hering-Junghans is a Group Leader at the Leibniz Institut für Katalyse e.V. (LIKAT) in Rostock, Germany.
Robert Kretschmer is a Professor of inorganic chemistry at Chemnitz University of Technology, Germany.
Also of Interest