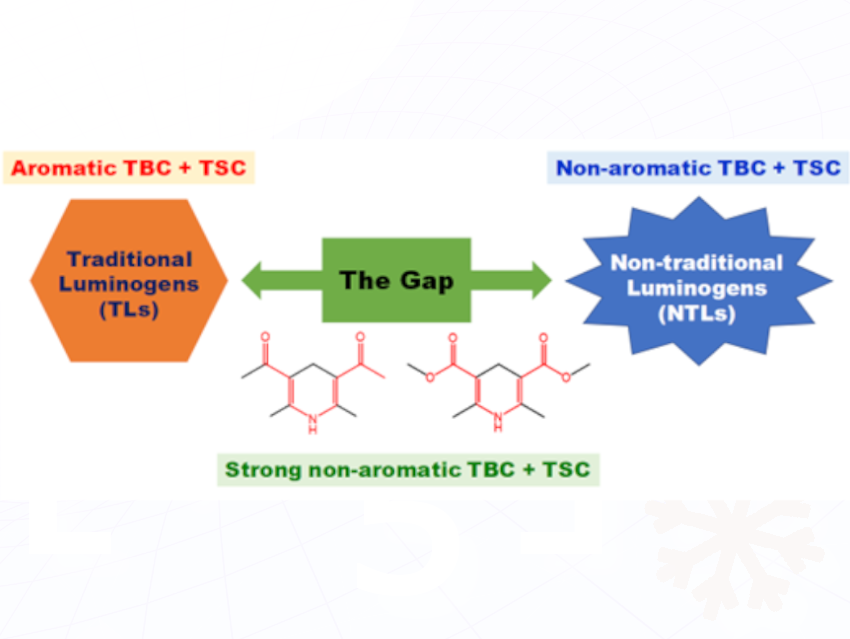
Through-bond conjugation (TBC) of conjugated aromatic groups plays an important role in the photoluminescence (PL) of traditional luminogens (TLs). In fact, intra/intermolecular through-space conjugation (TSC) effect has also been found to have a significant impact on the PL emission of TLs. The research on the PL mechanism of nontraditional luminogens (NTLs) mostly focuses on the intramolecular/intermolecular TSC of non-conventional chromophores.
Huiliang Wang, Beijing Normal University, Beijing, China, and colleagues designed and synthesized four dihydropyridine derivatives with strong non-aromatic TBC and studied the effects of non-aromatic TBC and TSC on the photophysical properties of the NTLs. These compounds in solutions show significant concentration-dependent and excitation-dependent emissions, which are typical PL behaviors of NTLs. In solid state, the compounds show wide excitation spectra while narrow emission spectra, with high quantum yields up to 57.4%, but they do not show significant excitation-dependent emissions, similar to TLs. And very impressively, two kinds of crystals also exhibit optical waveguide property, which is the first report in NTLs. The UV-vis spectra, crystal structures and theoretical calculations prove the presence of large non-aromatic TBC interactions in these NTLs and strong non-aromatic TSC can be formed among the molecules which are in a planar conformation and stacked into layers through intermolecular hydrogen bonding and π×××π interactions. The combined effect of strong non-aromatic TBC and TSC endow the compounds unique PL behaviors that are between those of TLs and NTLs, thus bridging the gap between TLs and NTLs.
This study bridges the gap between TLs and NTLs, and provides a deeper understanding of the PL mechanism of organic luminogens.
- Bridging the gap between traditional and nontraditional luminogens with strong non-aromatic through-bond conjugation and through-space conjugation
Xiaomi Zhang, Yunhao Bai, Junwen Deng, Xuanshu Zhong, Jinsheng Xiao, Wendi Xie, Huiliang Wang
Aggregate 2025
https://doi.org/10.1002/agt2.70063