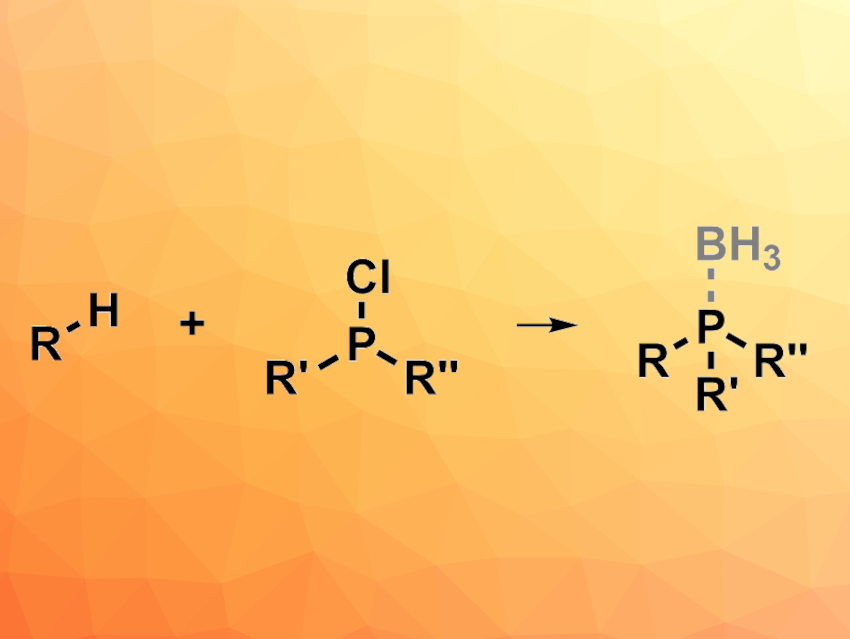
Tertiary phosphines can be useful as reactants, ligands, or organocatalysts in, e.g., organic synthesis and coordination chemistry. However, the synthesis of unsymmetrical tertiary phosphines, in particular, can still be challenging. Existing methods for this can have drawbacks such as a limited scope, low yields, and inconvenient operation. New approaches to the preparation of these species are, thus, interesting research targets. Radical phosphorylation reactions have shown promise for this type of transformation.
Feng-Cheng Jia, Wuhan Institute of Technology, China, Xiao-Qiang Hu, South-Central Minzu University, Wuhan, China, and colleagues have used an FeCl3-mediated ligand-to-metal charge transfer (LMCT) process for a radical phosphorylation in the presence of industrial phosphine sources (i.e., Ph2PCl or PCl3) to achieve C(sp3)–H bond phosphorylations (general reaction pictured). The team reacted a variety of compounds that feature C(sp3)–H bonds with Ph2PCl in the presence of FeCl3 under irradiation by 370 nm LED lights, using CH3CN as the solvent. To stabilize the resulting air-sensitive tertiary phosphines, they added borane after the phosphorylation reaction to form phosphine-borane complexes, which were isolated. PCl3 could also be used as a phosphine source to give dialkyl-substituted phosphines, which was followed by workup with H2O2 to obtain phosphine oxides.
The researchers propose a mechanism for the formation of tertiary phosphines that involves the excitation of the FeCl3 catalyst under LED irradiation, followed by the creation of a chlorine radical and an Fe(II) species via a photoinduced LMCT process. Then, a hydrogen-atom transfer (HAT) between the chlorine radical and a C(sp3)–H bond generates an alkyl radical and HCl. The catalyst is regenerated by a single electron transfer (SET) between a chlorophosphine and the Fe(II) intermediate, which gives a diphenylphosphinyl radical. Then, the diphenylphosphinyl radical can undergo a self-coupling to give a biphosphine compound, which reacts with the alkyl radical to give the desired product. Alternatively, the alkyl radical and the diphenylphosphinyl radical could undergo a coupling reaction to give the product.
- Radical Phosphorylation of Aliphatic C–H Bonds via Iron Photocatalysis,
Guang-Da Xia, Zi-Kui Liu, Yu-Lian Zhao, Feng-Cheng Jia, Xiao-Qiang Hu,
Org. Lett 2023.
https://doi.org/10.1021/acs.orglett.3c01824