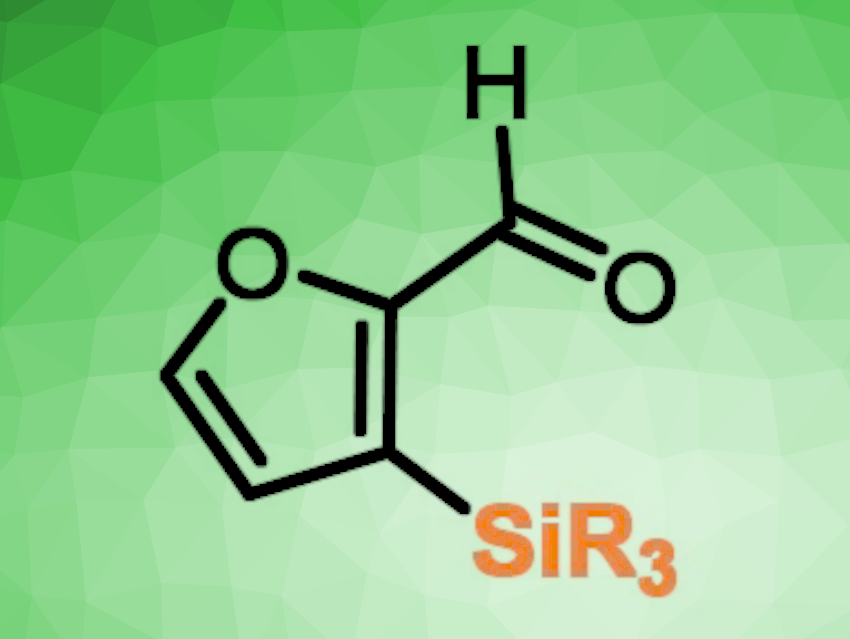
Furfural derivatives are among the most promising biobased molecules. They have great potential as a renewable platform for the sustainable production of fine chemicals or biofuels. The direct functionalization of furfural derivatives by selective C–H activation is, thus, an interesting research target.
Julie Oble, Sorbonne University, Paris, France, and colleagues have developed a method for the derivatization of furfurals in the C3-position (pictured below). Taking advantage of the aldehyde group, they first installed a temporary imine directing group. This directing group then allowed the regioselective C3-silylation of the furan ring.
The team reacted the imines with different hydrosilanes in the presence of 3,3-dimethylprop-1-ene, using [IrCl(COD)]2 (COD = cyclooctadiene) as the catalyst, N,N-diisopropylethylamine (DIPEA) as a base, and n-hexane as the solvent. The silylation was followed by hydrolysis of the imine using a HCl solution.
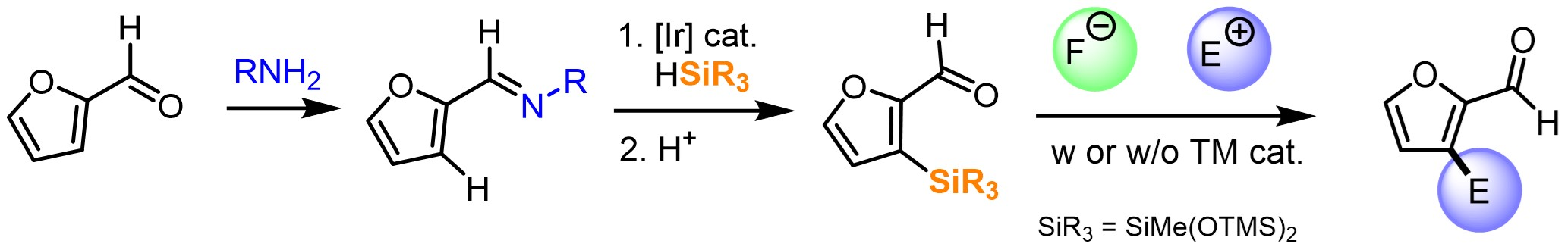
The products of the silylation can be further functionalized using the C(sp2)–Si bond as a handle (pictured above on the right). Strategies involving a fluoride-mediated activation of the C(sp2)–Si bond allow for a wide range of post-functional transformations, i.e., arylation, alkenylation, alkynylation, allylation, alkylation, halogenation, and trifluoromethylation. This range is promising for accessing value-added products from biomass-based substrates.
- C3–H Silylation of Furfural Derivatives: Direct Access to a Versatile Synthetic Platform Derived from Biomass,
Sébastien Curpanen, Giovanni Poli, Alejandro Perez Luna, Julie Oble,
Asian J. Org. Chem. 2022.
https://doi.org/10.1002/ajoc.202200199