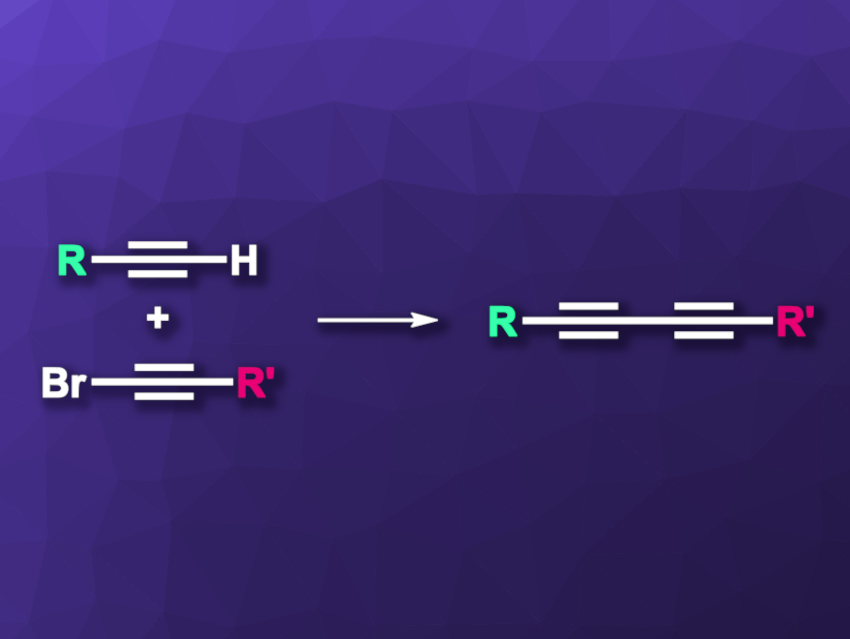
In Cadiot–Chodkiewicz cross-coupling reactions, a terminal alkyne and a 1-haloalkyne react under copper(I) catalysis to give an unsymmetric buta-1,3-diyne (general reaction pictured). Generally, these reactions require an inert atmosphere, because under air, the catalytically active Cu(I) species can be oxidized to Cu(II). This leads to unwanted side reactions that give symmetric buta-1,3-diynes, i.e., homo-coupling products, instead of the desired species. The yield is reduced and isolating the desired product in pure form becomes more difficult.
Michael S. Sherburn, Australian National University, Canberra, Australia, and colleagues have proposed that sodium ascorbate, i.e., the sodium salt of vitamin C, could serve as a Cu(II) → Cu(I) reducing agent and prevent the unwanted side reactions that can be caused by exposure to air. The team reacted a variety of both terminal alkynes and 1-bromoalkynes in the presence of CuBr as a catalyst, n-BuNH2 as a base, sodium ascorbate as a reductant, and ethanol as the solvent. The reactions were performed in air and at room temperature.
The desired cross-coupling products (23 examples) were obtained in high yields. Several products that are usually obtained in low yields in Cadiot–Chodkiewicz cross-coupling reactions showed significantly improved yields.
The team extended the method to Sonogashira cross-coupling reactions: They successfully performed high-yield reactions between iodobenzene and terminal alkynes in the presence of ascorbic acid under air, albeit in screw-capped vials instead of open to the air to limit exposure. Overall, the developed protocols are convenient, and the approach could possibly be further extended to other reactions.
- Air Tolerant Cadiot–Chodkiewicz and Sonogashira Cross-Couplings,
Alfred K. K. Fung, Madison J. Sowden, Michelle L. Coote, Michael S. Sherburn,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03314