Calcium ions are essential for cells but can be toxic in higher concentrations. Xiaoding Lou, China University of Geosciences, Wuhan, China, Juyoung Yoon, Ewha Womans University, Seoul, Republic of Korea, and colleagues have developed a combination drug that kills tumor cells by modulating the calcium influx into the cell. An external calcium source is not necessary because calcium ions already present in the tumor tissue are used.
“Calcium Storm” against Tumor Cells
Biological cells need calcium ions, among other things, for the proper functioning of the mitochondria. However, if there is too much calcium, the mitochondrial processes become unbalanced and the cell “suffocates” and dies.
The team took advantage of this process and developed a synergistic antitumor drug that can open calcium channels. This triggers a deadly calcium storm inside the tumor cell.
Dual Targets, Dual Calcium Sources
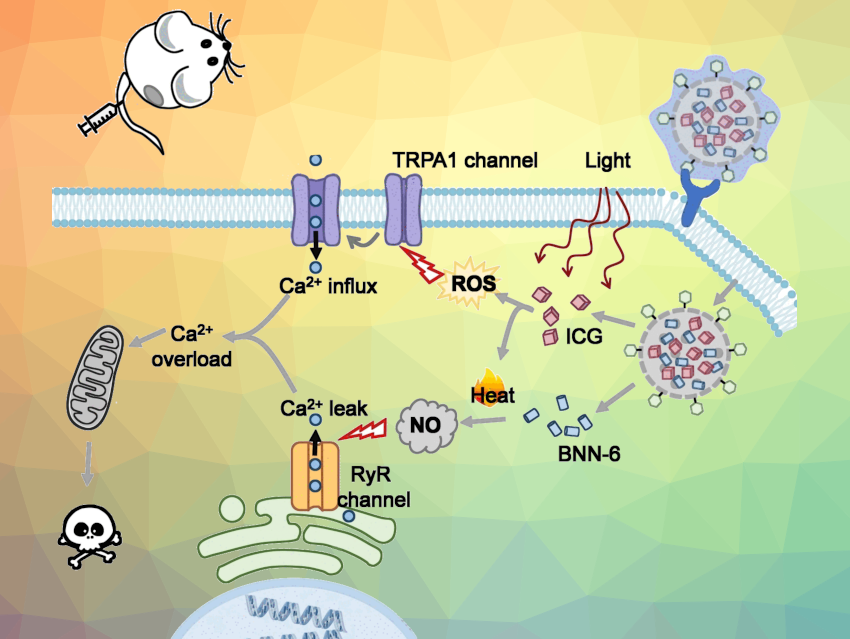
The researchers targeted two channels (pictured). The first one, the transient receptor potential ankyrin subtype 1 (TRPA1) protein, is found in the outer membrane. The second, a ryanodine receptor (RyR), is a calcium channel in the endoplasmic reticulum, a cell organelle that also stores calcium ions.
The channel located in the outer membrane opens when it is exposed to a large amount of reactive oxygen species (ROS). The channel in the endoplasmic reticulum is activated by nitric oxide (NO) molecules.
Photo-Controlled Activation
To generate the reactive oxygen species that open the outer membrane calcium channel, the researchers used the dye indocyanine green (ICG). This bioactive agent can be activated by irradiation with near-infrared (NIR) light, which not only triggers reactions that lead to ROS, but also heats the environment.
The high local temperature also activates the other active agent, the thermal-sensitive nitric oxide donor BNN-6 (N,N‘-di-sec-butyl-N,N‘-dinitroso-1,4-phenylenediamine), causing it to release NO molecules. The NO molecules then trigger the opening of the channel in the endoplasmic reticulum.
The two active agents were used together to build a photo-controlled Ca2+ nanomodulator. To create a biocompatible combined drug, the team loaded both ICG and BNN-6 into mesoporous silica nanoparticles. Then they coated the nanoparticles with hyaluronic acid, which targets tumor cells.
Success In Vivo
Following successful trials in tumor cell lines, the team tested an injectable formulation in tumor-implanted mice. After injecting the hybrid drug into the bloodstream of the mice, the researchers observed that the drug accumulated in the tumor. Exposure to near-infrared light successfully triggered the mechanism of action, and tumor growth was inhibited.
The researchers emphasize that this ion influx approach may also be useful in related biomedical research areas where a similar mechanism could activate different ion channels in order to find new therapeutic approaches.
- Photo‐Controlled Calcium Overload from Endogenous Sources for Tumor Therapy,
Jing‐Jing Hu, Lizhen Yuan, Yunfan Zhang, Jing Kuang, Wen Song, Xiaoding Lou, Fan Xia, Juyoung Yoon,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202317578