Nonsteroidal anti-inflammatory drugs (NSAIDs) are therapeutics with a wide range of pharmacological activities. They suppress the synthesis of prostaglandins by blocking the enzyme cyclooxygenase (COX). COX exists in two isoforms: COX-1 (“house-keeping” enzyme) and COX-2 (the induced isoform from pathological stimuli). COX-2 can be overexpressed in cancer cells. Thus, NSAIDs can also exhibit cytotoxic properties and promote cancer cell death by targeting this enzyme.
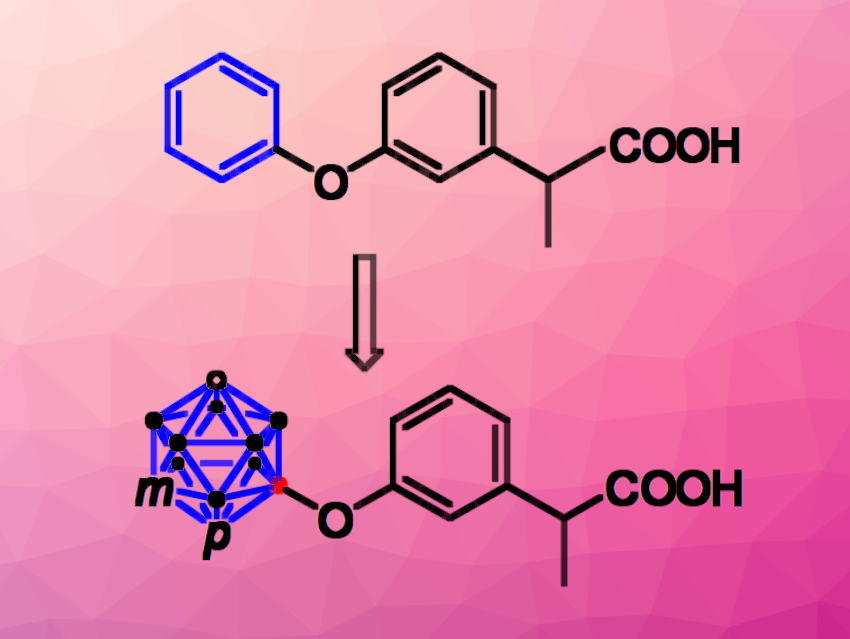
Fenoprofen (pictured at the top) is an NSAID used for the treatment of several diseases in which pain and inflammation are involved, such as rheumatoid arthritis, degenerative joint disease, or gout. It is a nonselective COX inhibitor showing only low cytostatic potential due to its low lipophilicity. Carboranes can be used as bulky, very hydrophobic biomimetic replacements of aryl groups, which could be useful to tune the properties of pharmaceutically active compounds such as fenoprofen.
Evamarie Hey-Hawkins, Leipzig University, Germany, and colleagues have synthesized carborane analogues of fenoprofen (pictured) to improve the biological properties of the drug. The team started from different carborane clusters, which were halogenated to introduce Br or I substituents. The resulting halo-carboranes underwent a palladium-catalyzed B–O coupling with racemic 2-(3-hydroxyphenyl)propionitrile to introduce the phenyl ring. Finally, the nitrile group was hydrolyzed to obtain the desired carboxylic acid derivatives.
The carborane-containing analogues show greater antitumor activity and COX-inhibition potential than fenoprofen. This strategy provides a promising approach to the development of new inorganic–organic hybrid molecules, e.g., for applications as antitumor agents, but also for other use cases.
- Carborane Analogues of Fenoprofen Exhibit Improved Antitumor Activity,
Liridona Useini, Marija Mojić, Markus Laube, Peter Lönnecke, Sanja Mijatović, Danijela Maksimović-Ivanić, Jens Pietzsch, Evamarie Hey-Hawkins,
ChemMedChem 2022.
https://doi.org/10.1002/cmdc.202200583