The cooperative assembly of hydrogen bond donors and acceptors produces nanometer-sized domains of secondary structure in proteins, such as α-helices and β-sheets. The motion of such domains relative to one another leads to a protein’s conformational landscape. For some proteins, incoming signals can perturb this conformational distribution and cause domain reorientation that can extend over several nanometers. This process is the basis for the allosteric regulation of enzymes, i.e., a change in the activity of the enzyme due to a conformational change induced by another molecule.
Artificial systems with similar responsive properties that can transmit signals over nanometer distances without the physical transport of a signaling molecule could have widespread applications. Combining a rigid scaffold and a conformationally switchable hydrogen-bonded array is an attractive approach towards building such systems.
Simon John Webb, University of Manchester, UK, and colleagues have created a scaffolded nanoscale array that responds to changes in a chemical signal and transmits them along the system. The team used a 1.8-nm-long rigid nanorod made from oligo(phenylene-ethynylene)s as scaffolds and attached multiple squaramides to form a switchable hydrogen-bonded relay. Squaramides (SQs) are vinylogous amides with a rigid planar structure, which contain both hydrogen-bond-donating NH units and hydrogen-bond-accepting carbonyl oxygens opposite to each other. In SQ-SQ pairs, the two units can have opposite orientations, with the first SQ acting either as a hydrogen-bond donor or as a hydrogen-bond acceptor. This orientation can be reversibly switched along the array.
Transfer of Conformational Information
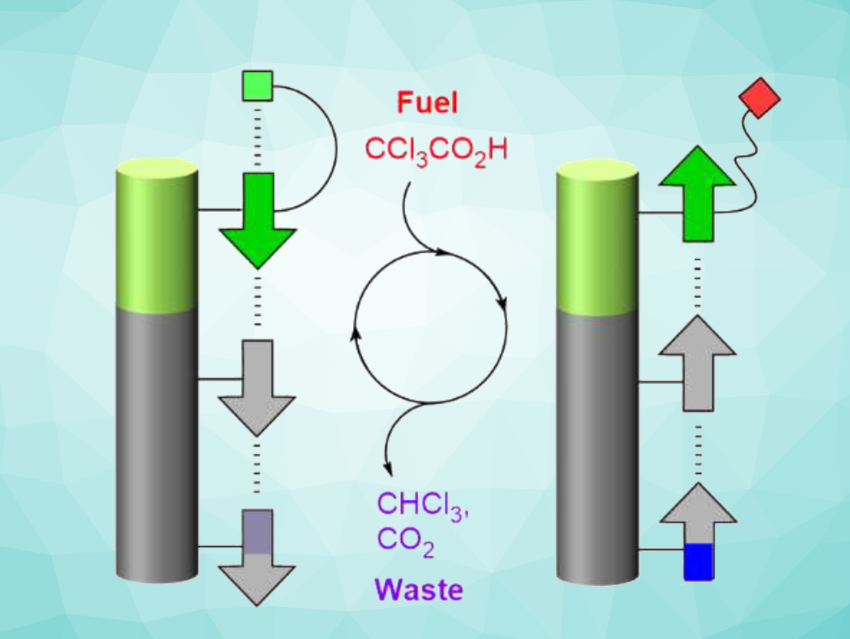
A “director” group is added to one end of the system to induce a preferred orientation in the absence of an additional signal, and a “reporter” group at the other end is used to “read out” the configuration of the squaramides by observing changes in its 1H NMR spectrum. When the director group is switchable by an external signal, this signal can induce reversible changes in the orientation of the squaramides, which are “reported” at the other end of the nanorods. The team used an amine-containing director group, which responds to proton signals and can lead to multiple reversible changes in orientation when acids or bases are added.
With such a group that is acid-base switchable, the addition of the “molecular fuel” CCl3COOH can be used to push the relay into an out-of-equilibrium state, which then relaxes over time as the fuel is consumed (pictured above). Overall, the work provides a new method for relaying chemical information over a multi-nanometer distance.
- Chemically Fueled Communication Along a Scaffolded Nanoscale Array of Squaramides,
Luis Martínez-Crespo, Iñigo J. Vitórica-Yrezábal, George F. S. Whitehead, Simon John Webb,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202307841