Hydroborations of unsaturated compounds are useful tools in organic synthesis. There are different hydride reagents that can be used for this, with different drawbacks such as limited substrate scope, limited selectivity, or safety concerns. Transition-metal catalysts can help to address these issues but can be costly. Catalysts based on abundant elements and simple ligands would be useful in this context.
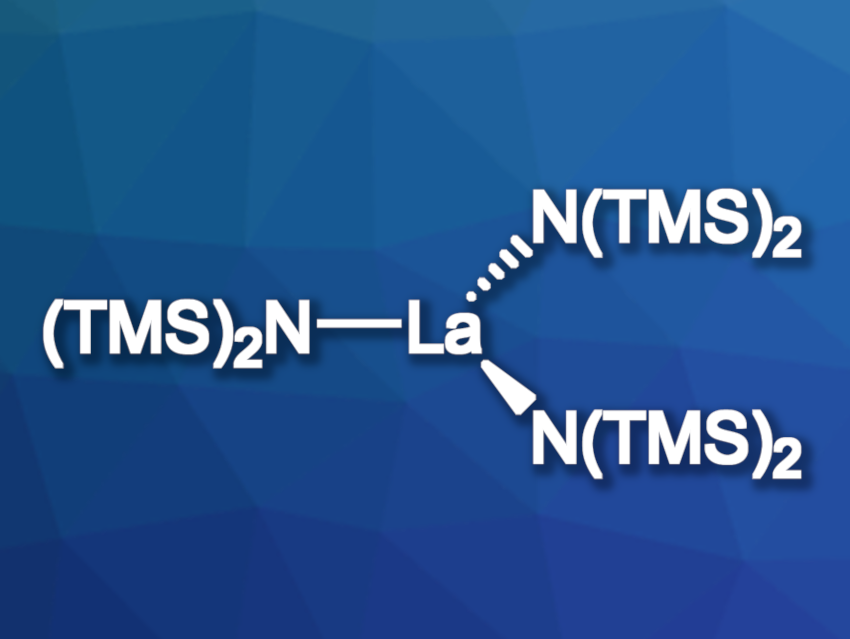
Yosi Kratish and Tobin J. Marks, Northwestern University, Evanston, IL, USA, Alessandro Motta, Universitá di Roma “La Sapienza”, Rome, Italy, and colleagues have investigated the reaction mechanisms of aldehyde and ketone hydroborations with pinacolborane (HBpin) catalyzed by La[N(SiMe3)2]3 (pictured). The team used NMR spectroscopy, elemental analysis, and X-ray crystallography to characterize the involved structures, as well as density functional theory (DFT) calculations to understand the possible reaction mechanisms.
The researchers found that the carbonyl oxygen of the ketones or aldehydes binds to the Lewis-acidic La center to initiate the reaction, followed by an intramolecular ligand-assisted hydroboration of the carbonyl moiety by bound HBpin. The formation of a bidentate acylaminato La complex in the case of aldehyde substrates involves an interaction of the ligand with the substrate and indicates that the N(SiMe3)2 ligands have a chemically non-innocent role in the reactions.
- Mechanistic study of homoleptic trisamidolanthanide-catalyzed aldehyde and ketone hydroboration. Chemically non-innocent ligand participation,
Jacob O. Rothbaum, Alessandro Motta, Yosi Kratish, Tobin J. Marks,
Chem. Sci. 2023.
https://doi.org/10.1039/D2SC06442A