1 Exploding Trousers
1.1 Mysterious Events
On August 12, 1931, a local newspaper in New Zealand called the “Hawera Star” shocked its readers with the following news [1]: “While Mr. Richard Buckley’s trousers were drying before the fire recently, they exploded with a loud report. Although partially stunned by the force of the explosion, he had sufficient presence of mind to seize the garments and hurl them from the house, where they smoldered on the lawn with a series of minor detonations.”
More incidents were also reported in other regions of New Zealand: A farmer suffered a serious shock when a pair of trousers she had hung out on the line went up in flames after drying. Numerous farm workers learned the bitter truth that smoking is unhealthy when their clothing suddenly caught fire as they lit their cigarettes. While out on a ride, a farmer’s pants began to smolder in the saddle [2].
Not everyone got off lightly, especially if the wearer was still in the trousers. The worst case was that of a farm worker who wanted to look at his sleeping baby at the end of the day. Because his lodgings had no electric light, he lit a match to better be able to see his little child. His clothes burst into flame and he died a short time later from his injuries.
The culprit behind these tragic incidents was quickly found to be sodium chlorate, NaClO3. This substance had been employed extensively as a weed killer (herbicide) in New Zealand agriculture since 1930. For this application, the salt was dissolved in water and sprayed directly onto the “weeds”.
The term “weed” is used colloquially to refer to an unwanted plant. The meaning, thus, depends on the location of the plant and the observer. Cornflowers and field poppies are wonderful for hikers to look at but are “weeds” when they pop up in a farmer’s wheat field. For the farmers of New Zealand, ragwort was not merely undesirable in their pastures, its toxicity to their animals made it a threat to their existence.
Because the farm laborers did not wear any protective covering, their cotton clothing became fully saturated with the sodium chlorate solution over the course of the workday. Once dry, a single spark or a little heat from friction was all it took to ignite the fibers of the material, which acted like fuses.
But why did the farmers spray their fields with aqueous sodium chlorate solutions? To address this question, we need to take a closer look at the development of the agrarian economy of New Zealand.
 |
|
Figure 1. Ragwort. Left: two-year-old ragwort (Jacobaea vulgaris, Syn. Senecio jacobaea) is in the Asteraceae family (Image source: Christian Fischer, wikimedia commons, CC BY-SA 3.0). |
1.2 Disturbed Ecological Balance
In New Zealand, sheep farming was dominant at first. The animals ate plants growing in the meadows, such as grasses, clover, and ragwort (Senecio jacobaea, also called stinking willie because of the revolting odor of its crushed leaves), which had been imported from Europe (see Fig. 1) [3]. This ecological equilibrium started to unravel in the early 20th century, as new cooling technology combined with motorization revolutionized the agricultural economy of New Zealand.
With these changes, agricultural products could easily be transported over longer distances. This made the dairy industry financially attractive for the mostly family-run farms because dairy processors were able to pick up milk from remote farms with refrigerated trucks. Between 1900 and 1940, the number of dairy cows quadrupled, while the number of sheep declined. This development inadvertently led to a change in the vegetation in the meadows because unlike sheep, cattle and horses avoid eating ragwort. The unpleasant odor and bitterness of the plant probably deterred the animals from eating it. In consequence, ragwort slowly crowded out the grasses and clover [4].
In 1926, a comprehensive report from the New Zealand state biological research institute in Wellington identified this existential danger and pointed out the high—often fatal—toxicity of ragwort to cattle and horses [5]. Ragwort poisoning in horses and cows occurs all over the world: as Winton disease in New Zealand, Schweinsberg disease in Germany, Zdam horse plague in the Czech Republic, walking disease in the USA, Pictou cattle disease in Canada, and stiff sickness in South Africa. While cattle and horses do avoid bitter ragwort, young plants contain small amounts of the bitter substances but very high levels of toxins. When food became scarce, the animals were forced to also eat the toxic plants.
Feeding the animals with hay and silage was problematic because, in these feeds, the bitter substances that act as a warning have broken down, but the toxins have not (see Infobox).
Infobox: The Toxins in Ragwort
(click here to expand)
Like many plants, ragwort defends itself from predators with chemical weapons, specifically with pyrrolizidine alkaloids [6]. It is estimated that about 6,000 plant species produce pyrrolizidine alkaloids.
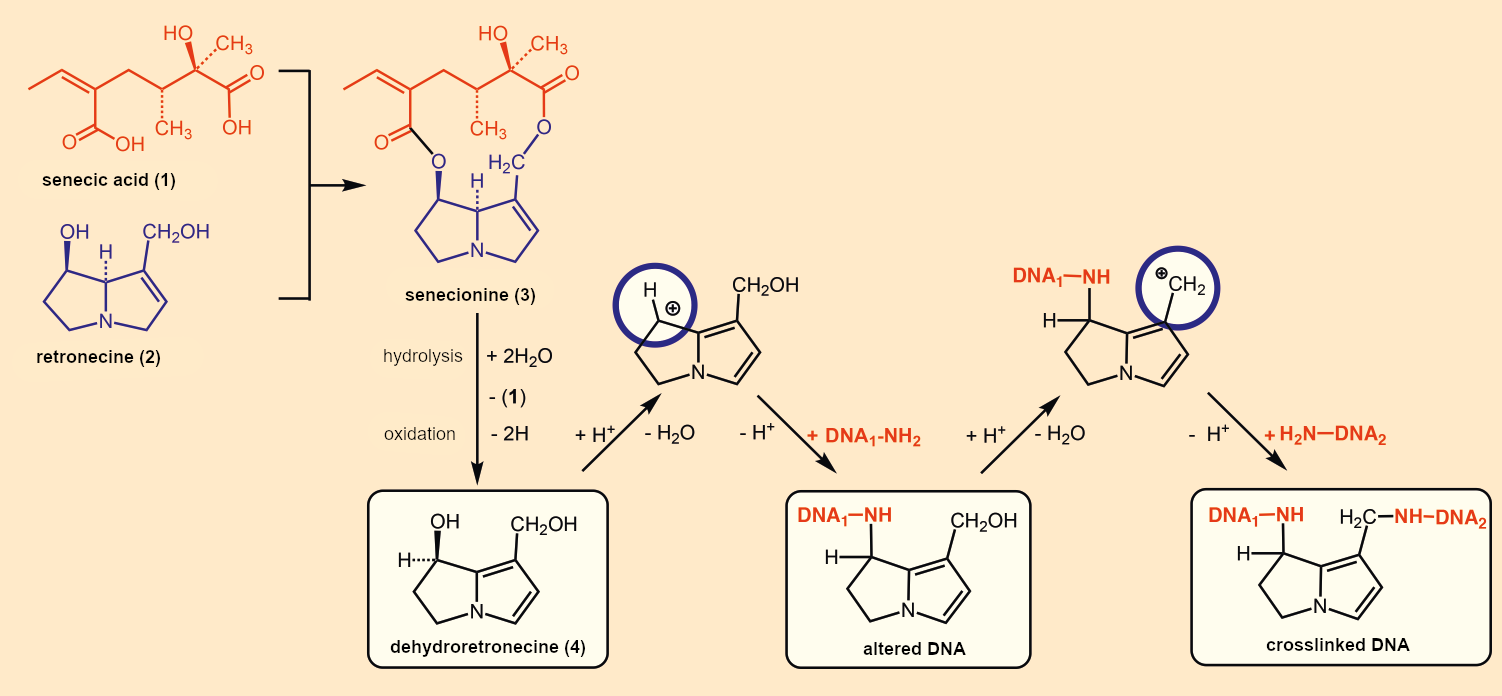
The chemical synthesis of pyrrolizidine alkaloids is illustrated in Figure B1 for senecionine (3): A double esterification of a dicarboxylic acid (1, senecic acid) with a pyrrolizidine alkaloid (2, retronecine) leads to the alkaloid (3). Through slight structural variations of the acid and alkaloid components, ragwort prepares a whole alkaloid poison cocktail for its defense.
 |
|
Figure B1. Structure and toxicity of pyrrolizidine alkaloids. |
It is not the substance in the plant itself, senecionine (3), that is toxic; it is the dehydroretronecine (4) that is formed in the liver of mammals. Genetic damage occurs because two relatively stable cations (blue circles) can be formed from (4). These can attack free NH2 groups of DNA and either block them or crosslink the DNA [7]. Many experiments indicate a causal relationship between chronic ingestion of these alkaloids and liver disease. Typical consequences include fatty liver, cirrhosis, venous occlusion in the liver and lungs, and liver tumors.
Mammals have different reactions to ragwort. Sheep are relatively impervious; cattle and horses are more sensitive [8]. Humans are actually very sensitive [7,9]! Because ragwort can also enter the human food chain, the German Federal Institute for Risk Assessment (Bundesinstitut für Risikobewertung, BfR) monitors the amount of pyrrolizidine alkaloids in foods with particular vigilance, especially because many people are convinced that Nature is always good to us. Ragwort teaches us that this is not always true. Years of demands made by the BfR to producers and dealers have recently paid off: The BfR, fortunately, determined that the contamination of honey and herbal teas with pyrrolizidine alkaloids is declining [10].
1.3 Sodium Chlorate in Agriculture
Until 1930, the growth of ragwort and associated loss of animals increased steadily. The farmers of New Zealand sought help from their Ministry of Agriculture. Initial attempts to kill off the weed with biological methods failed—both the release of moths (cinnabar moth, Tyria jacobaeae) in 1929 and flies (ragwort seed fly, Botanophila senciaella) in 1940 [11]. (It was not until the 1960s and 1970s that the simultaneous implementation of the cinnabar moth (Tyria jacobaeae) and the tansy ragwort flea beetle (Longitarsus jacobaeae) in California brought great success. However, ragwort remains a problem [12].)
The use of sodium chlorate was suggested to the farmers of New Zealand [13], and it was promised that “100 percent eradication will be achieved if the plants are sprayed correctly” (see Fig. 2). In their desperation, dairy farmers immediately took this suggestion, as shown by national import figures: Imports of sodium chlorate rose from 0 in 1930 to 1000 tons by 1937.
 |
|
Figure 2. Sodium chlorate against ragwort. Left: Precisely localized application of the aqueous sodium chlorate solution reduced contact between the solution and clothing. (Image source: advertisement in the “New Zealand Farmer” (Feb. 1, 1933), public domain). Center: advertisement for “Unkraut-Ex” (“Weed-Ex”) from 1938. Right: advertisement for “Wegerein”, from 1958 by the Bitterfeld Electrochemical Combine. |
After a short time, more incidents like Richard Buckley’s began to crop up due to improper handling of sodium chlorate, requiring the subsequent implementation of rules for its use [14]. In the long term, sodium chlorate was not successful on a large scale because the treatment of individual plants was labor-intensive, and transportation and safe storage and handling were too dangerous for laypeople. Since then, there have been no more exploding trousers, and the use of sodium chlorate in New Zealand became a forgotten historical footnote. Only when James Watson at the Massey University Palmerston North, New Zealand, published a study entitled “The significance of Mr. Richard Buckley’s Exploding Trousers” in 2004 was attention turned to these tragic incidents [2]. Watson was awarded the Ig-Nobel Prize for Agricultural History in 2005.
Despite their danger, chlorates are still produced on an industrial scale and play an important, mostly unappreciated role in our everyday lives. This merits a closer look. Regardless, it remains true that there are many chlorate explosions along the way.
References
[1] Hawera Star, August 12, 1931.
[2] J. Watson, The Significance of Mr. Richard Buckley’s Exploding Trousers: Reflections on an Aspect of Technological Change in New Zealand Dairy Farming between the World Wars, Agric. Hist. 2004, 78, 346–360.
[3] Landesverband Westfälischer und Lippischer Imker e.V., Hamm, Deutschland, Jakobskreuzkraut Infoblatt, www.lv-wli.de. (accessed October 3, 2022)
[4] V. Sesin, J. R. Freeland, C. M. Davy, Protecting plant communities with weed killers, Nachr. Chem. 2021, 69, 75–78. https://doi.org/10.1002/nadc.20214116549
[5] E. Atkinson, Ragwort (Senecio Jacobea) and its Relation to Winton Disease, New Zealand J. Agric. 1926, 33, 159.
[6] E. Petzinger, Pyrrolizidinalkaloide und die Seneciose bei Tieren – Teil 1: Vorkommen, Chemie, Toxikologie, Tierärztl. Prax. G 2011, 39, 221–230. https://doi.org/10.1055/s-0038-1623063
[7] M. Lechtenberg et al., Hepatotoxisch und karzinogen: Pyrrolizidinalkaloide in Arznei- und Lebensmitteln sorgen für Probleme, Deutsche Apotheker Zeitung 2017, 31, 32.
[8] E. Petzinger, Pyrrolizidinalkaloide und die Seneciose bei Tieren – Teil 2: Klinik, Speziesunterschiede, Rückstandsverhalten, Futtermittelkontamination und Grenzwerte, Tierärztl. Prax. 2011, 39, 363–372. https://doi.org/10.1055/s-0038-1623090
[9] P. P. Fu et al., Genotoxic Pyrrolizidine Alkaloids — Mechanisms Leading to DNA Adduct Formation and Tumorigenicity, Int. J. Mol. Sci. 2002, 3, 948–964.
[10] Bundesinstitut für Risikobewertung, Aktualisierte Risikobewertung zu Gehalten an 1,2-ungesättigten Pyrrolizidinalkaloiden (PA) in Lebensmitteln, Stellungnahme Nr. 026/2020 des BfR vom 17. Juni 2020. https://doi.org/10.17590/20200617-130910
[11] S. V. Fowler et al., Biological control of ragwort in the New Zealand dairy sector: an ex-post economic analysis, New Zealand J. Agr. Res. 2016, 59, 205–215. https://doi.org/10.1080/00288233.2016.1170050
[12] Stiftung Naturschutz Schleswig-Holstein, Umgang mit dem Jakobs-Kreuzkraut: Meiden – Dulden – Bekämpfen, 2017. ISBN: 978-3-937937-88-5
[13] J. W. Deem, Control of Ragwort and Other Weeds by Spraying: Remarkable Results with Sodium and Calcium Chlorate, New Zealand J. Agric. 1930, 40, 291–294.
[14] B. C. Aston, J. A. Bruce, The Chemistry of Weed-Killers, New Zealand J. Agric. 1933, 47, 230–232.
The article has been published in German as:
- Als Richard Buckleys Hose explodierte,
Klaus Roth,
Chem. unserer Zeit 2022, 56, 102–109.
https://doi.org/10.1002/ciuz.202100062
and was translated by Caroll Pohl-Ferry.
Chlorates: Tragic Incidents and Life-Saving Applications – Part 2
Historic chlorate explosions and modern accidents
Chlorates: Tragic Incidents and Life-Saving Applications – Part 3
A vital use of chlorates as a source of oxygen—for example, in airplanes
See similar articles by Klaus Roth published on ChemistryViews.org